Abstract
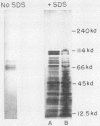
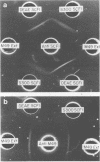
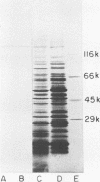
The biochemical and immunological characteristics of the chemotactic factor inactivator of group A streptococci (SCFI) were examined. SCFI was extracted from intact M+ bacteria by limited trypsin digestion and purified sequentially by ammonium sulfate fractionation, hydrophobic interaction chromatography, and anion-exchange chromatography. SCFI activity was associated with multiple species as indicated by gel permeation chromatography and DEAE high-pressure liquid chromatography analyses. Polyacrylamide gel electrophoresis of the column-purified protein also demonstrated a high degree of molecular heterogeneity, with most of the material in a 103,000 to 114,000 Mr cluster. SCFI activity was sensitive to destruction by several proteolytic enzymes, and polyclonal antiserum to SCFI was able to neutralize its antichemotactic activity. Two-dimensional immunoelectrophoresis of SCFI indicated that the various copurified species were immunologically cross-reactive and indicated a high degree of antigenic homogeneity within the preparation. Western blot analysis of crude detergent extracts of M+ bacteria identified a major antigenic species corresponding to 135,000 Mr and a less abundant species of 137,000 Mr. SCFI antiserum was not reactive with M protein, and therefore SCFI appeared to be a distinct molecule, despite its close association with the M+ phenotype.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Shank R. P., Graham L. T., Jr, Aprison M. H., Werman R. Association of glycine with spinal interneurones. Nature. 1967 May 13;214(5089):680–681. doi: 10.1038/214680a0. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganrot P. O. Crossed immunoelectrophoresis. Scand J Clin Lab Invest Suppl. 1972;124:39–47. doi: 10.3109/00365517209102749. [DOI] [PubMed] [Google Scholar]
- Johnson R. H., Vosti K. L. Purification and characterization of group A streptococcal T-1 antigen. Infect Immun. 1977 Jun;16(3):867–875. doi: 10.1128/iai.16.3.867-875.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J Exp Med. 1952 Jul;96(1):83–97. doi: 10.1084/jem.96.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T. Y., NEUMANN N. P., ELLIOTT S. D., MOORE S., STEIN W. H. Chemical properties of streptococcal proteinase and its zymogen. J Biol Chem. 1963 Jan;238:251–256. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwicka A., Kłoczewiak M. Characterization of group A streptococcal T-12 protein purified by ion-exchange column chromatography. Infect Immun. 1978 Sep;21(3):940–945. doi: 10.1128/iai.21.3.940-945.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Schmeling D., Cleary P. P., Wilkinson B. J., Kim Y., Quie P. G. Inhibition of alternative complement pathway opsonization by group A streptococcal M protein. J Infect Dis. 1979 May;139(5):575–585. doi: 10.1093/infdis/139.5.575. [DOI] [PubMed] [Google Scholar]
- Weis J. J., Law S. K., Levine R. P., Cleary P. P. Resistance to phagocytosis by group A streptococci: failure of deposited complement opsonins to interact with cellular receptors. J Immunol. 1985 Jan;134(1):500–505. [PubMed] [Google Scholar]
- Wexler D. E., Nelson R. D., Cleary P. P. Human neutrophil chemotactic response to group A streptococci: bacteria-mediated interference with complement-derived chemotactic factors. Infect Immun. 1983 Jan;39(1):239–246. doi: 10.1128/iai.39.1.239-246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]