Abstract
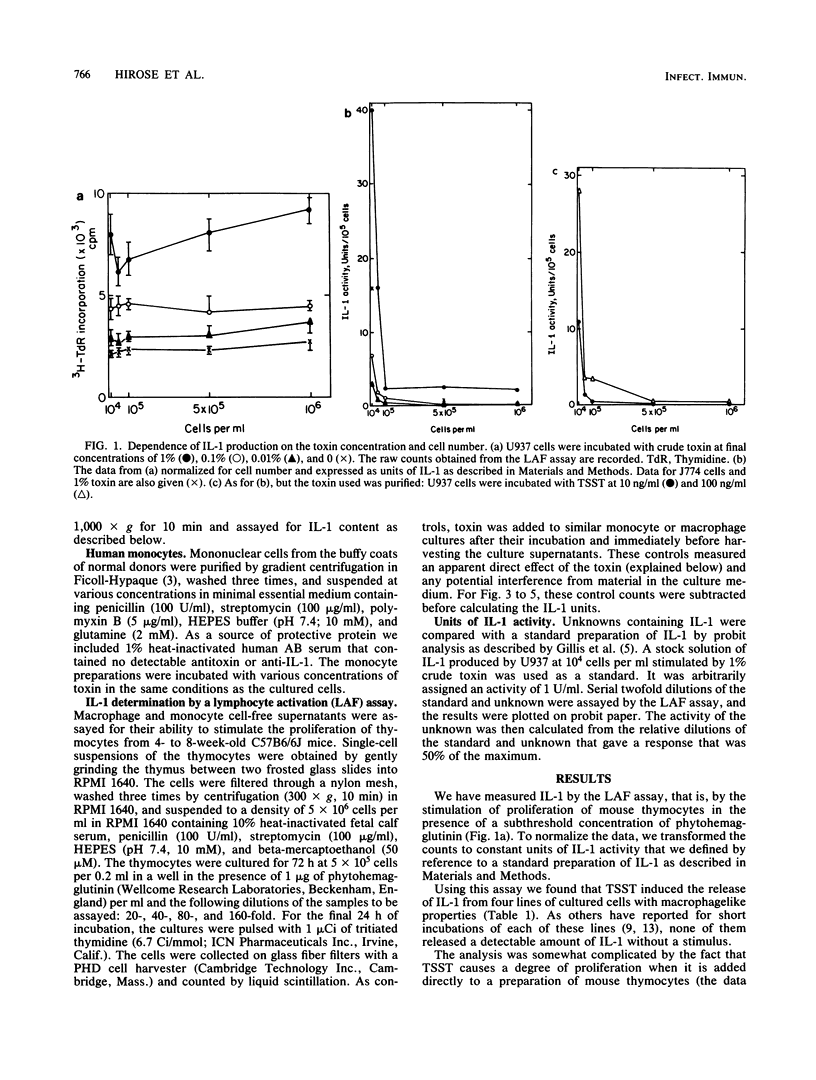
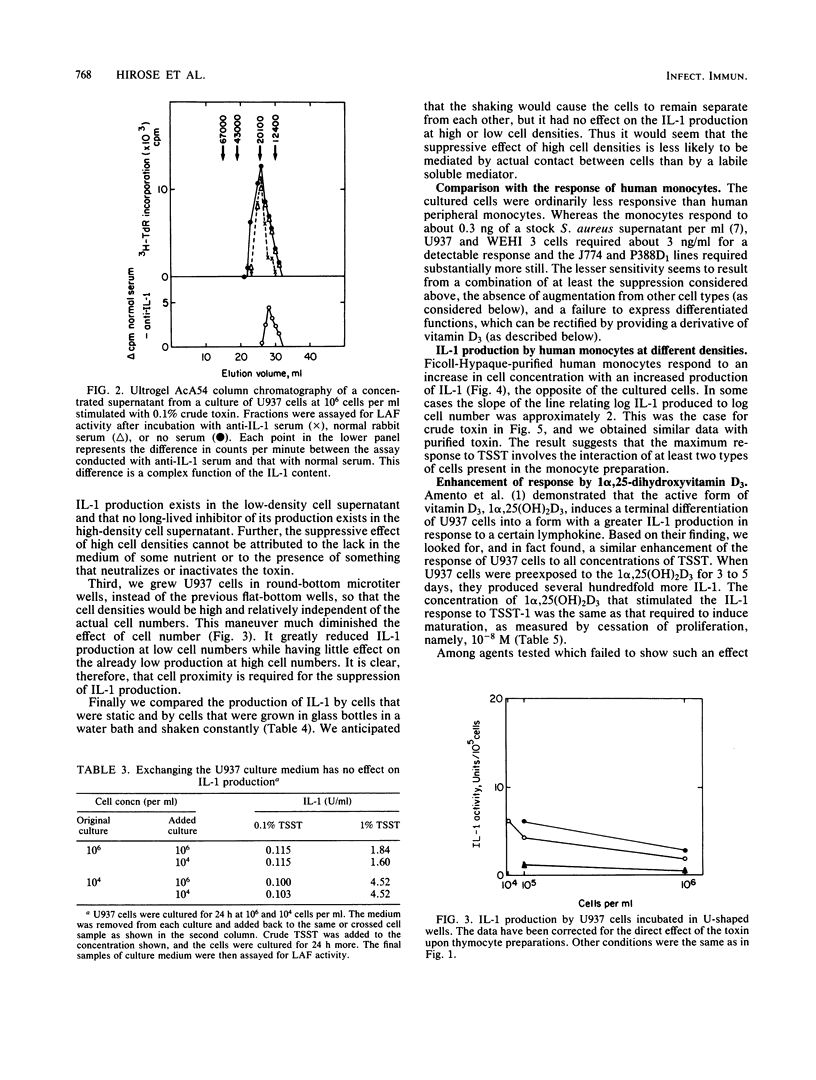
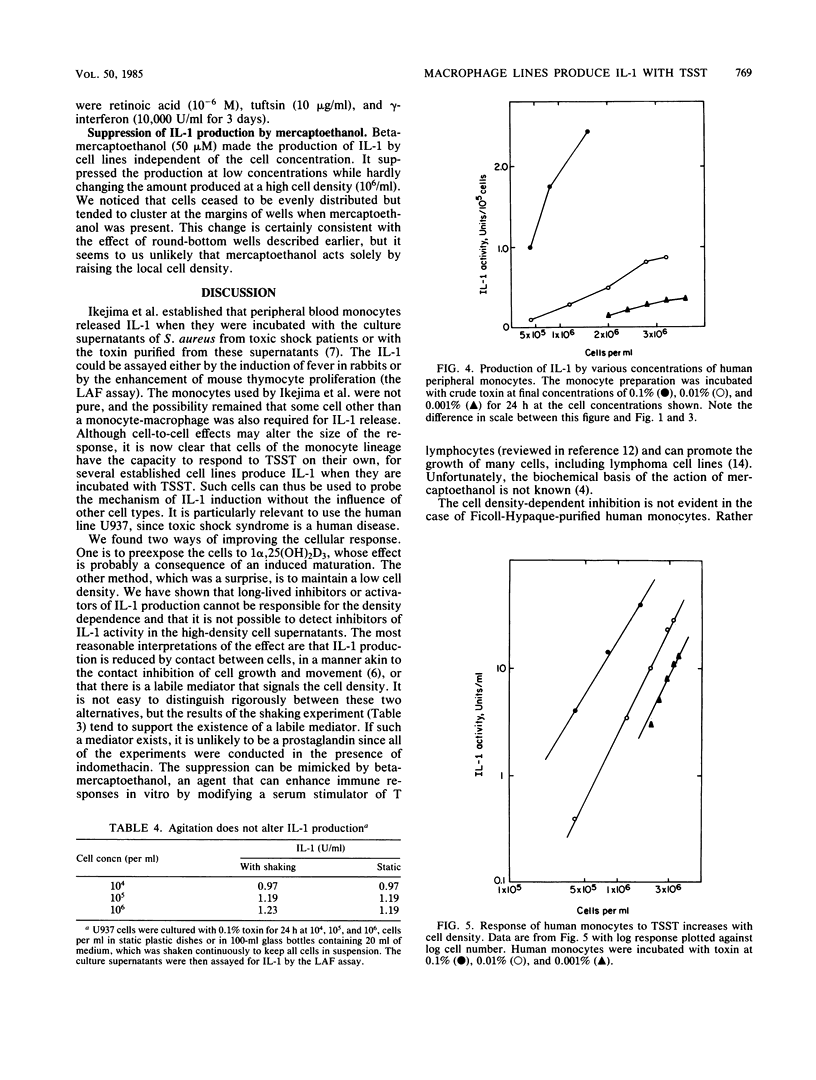
Toxic shock syndrome toxin is already known to induce the production of interleukin-1 (IL-1) by preparations of monocytes and macrophages that are presumably contaminated with other types of cells. The response is enhanced by increasing the density of such monocytes, suggesting that the monocyte's response to toxic shock syndrome toxin may be augmented by its interaction with some other cell. Nevertheless, we now show that several human and murine macrophagelike cell lines (U937, J774, P388D1, and WEHI 3) produce IL-1 when exposed to toxic shock syndrome toxin, and therefore the basic response does not require the presence of cells of other lineages. The cultured cells generally produce less IL-1 than do monocytes, but considerably more IL-1 is induced from cells that have undergone a terminal differentiation as a result of exposure to 1 alpha,25-dihydroxyvitamin D3. High concentrations of cultured cels suppress the production of IL-1; this effect is apparently not due to long-lived inhibitors of IL-1 production or of IL-1 activity, but may be due to a short-lived inhibitor of production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Bhalla A. K., Kurnick J. T., Kradin R. L., Clemens T. L., Holick S. A., Holick M. F., Krane S. M. 1 alpha,25-dihydroxyvitamin D3 induces maturation of the human monocyte cell line U937, and, in association with a factor from human T lymphocytes, augments production of the monokine, mononuclear cell factor. J Clin Invest. 1984 Mar;73(3):731–739. doi: 10.1172/JCI111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amento E. P., Kurnick J. T., Krane S. M. Interleukin 1 production by the human monocyte cell line U937 requires a lymphokine induction signal distinct from interleukin 2 or interferons. J Immunol. 1985 Jan;134(1):350–357. [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Lachman L. B., Hacker M. P., Blyden G. T., Handschumacher R. E. Preparation of lymphocyte-activating factor from continuous murine macrophage cell lines. Cell Immunol. 1977 Dec;34(2):416–419. doi: 10.1016/0008-8749(77)90263-5. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz H. G., Lemke H., Hewlett G. Activation of T-cells by a macrophage or 2-mercaptoethanol activated serum factor is essential for induction of a primary immune response to heterologous red cells in vitro. Immunol Rev. 1978;40:53–77. doi: 10.1111/j.1600-065x.1978.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Ivhed I., Sideras P., Nilsson K., Sugawara I., Fernandez C. Accessory function of human tumor cell lines. I. Production of interleukin 1 by the human histiocytic lymphoma cell line U-937. Eur J Immunol. 1982 Oct;12(10):895–899. doi: 10.1002/eji.1830121018. [DOI] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Khoe G. P., Bergdoll M. S. Purification and some physicochemical properties of toxic-shock toxin. Biochemistry. 1983 Aug 2;22(16):3907–3912. doi: 10.1021/bi00285a028. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tse H. Y., Schwartz R. H., Paul W. E. Cell-cell interactions in the T cell proliferative response. I. Analysis of the cell types involved and evidence for nonspecific T cell recruitment. J Immunol. 1980 Aug;125(2):491–500. [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]


