Abstract
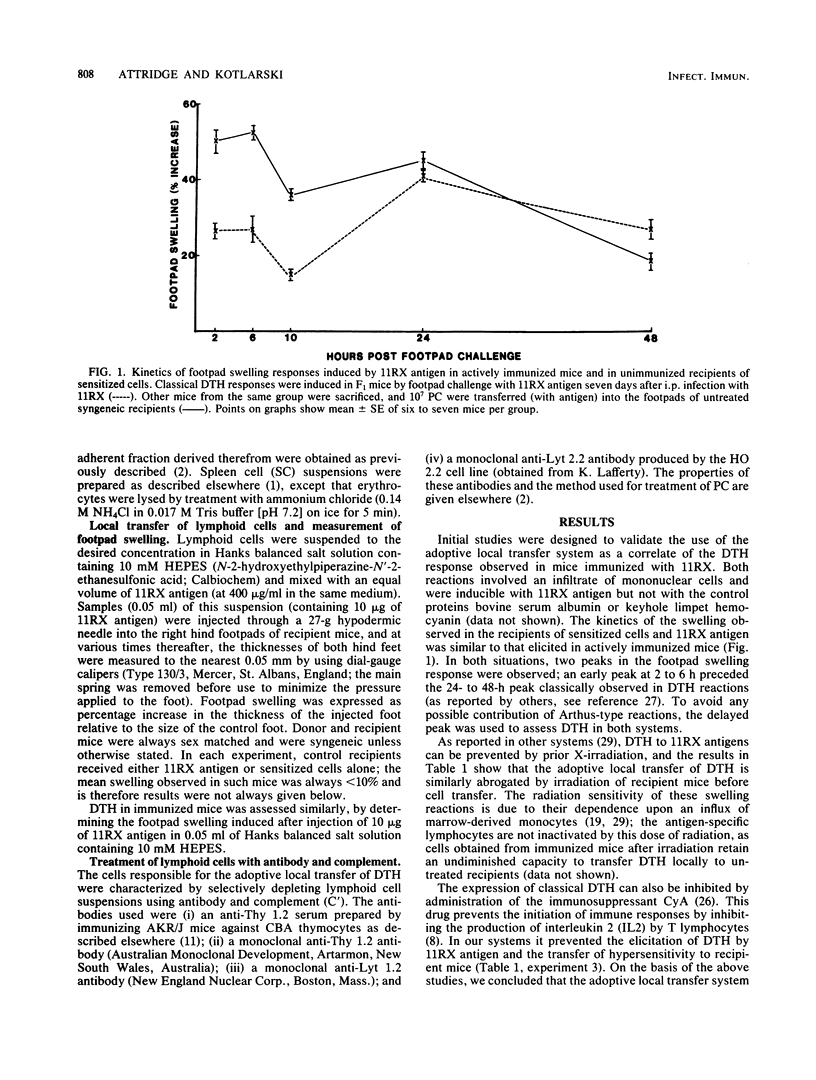
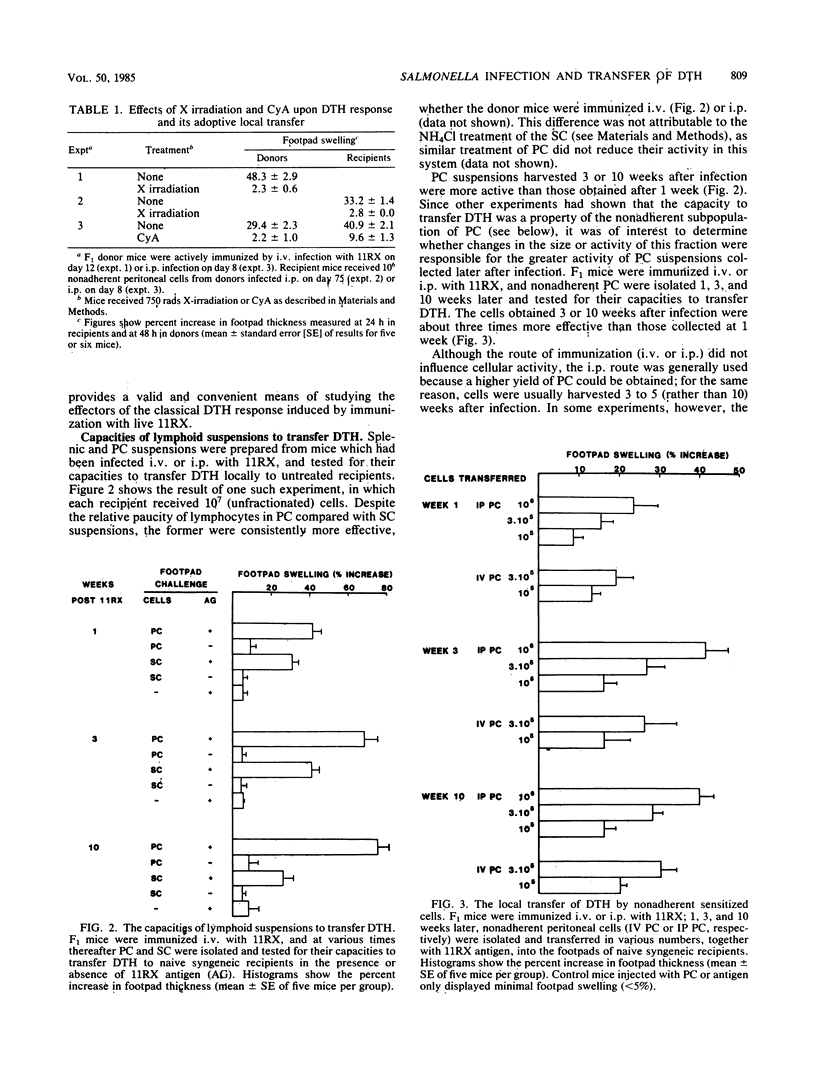
An adoptive local transfer system has been used to study the mediators of delayed-type hypersensitivity induced in mice by infection with Salmonella enteritidis 11RX. The cells which transfer this state of hypersensitivity to untreated recipients are nonadherent T lymphocytes with the surface phenotype Lyt 1+2-, and successful transfer requires compatibility at the I-A subregion of the H-2 complex. In these and other respects these cells are indistinguishable from those previously found to be responsible for in vitro lymphokine release upon culture with 11RX antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Kotlarski I. In vitro lymphokine release by lymphocytes from mice infected with Salmonella. Aust J Exp Biol Med Sci. 1985 Oct;63(Pt 5):489–502. doi: 10.1038/icb.1985.53. [DOI] [PubMed] [Google Scholar]
- Attridge S. R., Kotlarski I. The effects of Amicon and millipore membranes upon the lymphokine titres of culture supernatants. Aust J Exp Biol Med Sci. 1984 Oct;62(Pt 5):547–550. doi: 10.1038/icb.1984.52. [DOI] [PubMed] [Google Scholar]
- Cheers C., Sandrin M. S. Restriction in adoptive transfer of resistance to Listeria monocytogenes. II. Use of congenic and mutant mice show transfer to be H-2K restricted. Cell Immunol. 1983 Jun;78(2):199–205. doi: 10.1016/0008-8749(83)90274-5. [DOI] [PubMed] [Google Scholar]
- Chen-Woan M., McGregor D. D. The mediators of acquired resistance to Listeria monocytogenes are contained within a population of cytotoxic T cells. Cell Immunol. 1984 Sep;87(2):538–545. doi: 10.1016/0008-8749(84)90022-4. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Davies R., Kotlarski I. The effect of immunization with a rough strain of Salmonella enteritidis on the fate of Salmonella typhimurium in mice. Aust J Exp Biol Med Sci. 1974 Oct;52(5):779–789. doi: 10.1038/icb.1974.77. [DOI] [PubMed] [Google Scholar]
- Davies R., Kotlarski I. The role of thymus-derived cells in immunity to salmonella infection. Aust J Exp Biol Med Sci. 1976 Jun;54(3):221–236. doi: 10.1038/icb.1976.23. [DOI] [PubMed] [Google Scholar]
- Dos Reis G. A., Shevach E. M. Effect of cyclosporin A on T cell function in vitro: the mechanism of suppression of T cell proliferation depends on the nature of the T cell stimulus as well as the differentiation state of the responding T cell. J Immunol. 1982 Dec;129(6):2360–2367. [PubMed] [Google Scholar]
- Gill H. K., Dhaliwal J. S., Sukumaran K. D., Liew F. Y. Effect of irradiation on the precursor, activated and memory suppressor T cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Immunology. 1984 Dec;53(4):669–675. [PMC free article] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity. III. Effect of cyclophosphamide on the suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):172–176. doi: 10.1002/eji.1830080306. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Leuchars E., Davies A. J. Studies on immunological paralysis. VI. Thymic-independence of tolerance and immunity to type 3 pneumococcal polysaccharide. Cell Immunol. 1971 Dec;2(6):614–626. doi: 10.1016/0008-8749(71)90009-8. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Kunz H. W., Gill T. J., 3rd, Jungi R. Genetic control of cell-mediated immunity in the rat. II. Sharing of either the RT1.A or RT1.B locus is sufficient for transfer of antimicrobial resistance. J Immunogenet. 1982 Dec;9(6):433–443. doi: 10.1111/j.1744-313x.1982.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Effective antibacterial protection induced by a Listeria monocytogenes-specific T cell clone and its lymphokines. Infect Immun. 1983 Mar;39(3):1265–1270. doi: 10.1128/iai.39.3.1265-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Differences in delayed-type hypersensitivity responses in various mouse strains in the C3H lineage infected with Salmonella typhimurium, strain SL3235. J Immunol. 1984 Sep;133(3):1190–1196. [PubMed] [Google Scholar]
- Lefford M. J. Suppression of adoptive antituberculosis immunity by normal recipient animals. Infect Immun. 1983 Jul;41(1):257–263. doi: 10.1128/iai.41.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvik M., Closs O. Induction of delayed type hypersensitivity against ultrasonicated Mycobacterium lepraemurium bacilli without simultaneous local reactivity against live bacilli or protective immunity. Clin Exp Immunol. 1983 Aug;53(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Marchal G., Milon G., Hurtrel B., Lagrance P. H. Titration and circulation of cells mediating delayed type hypersensitivity in normal and cyclophosphamide treated mice during response to sheep red blood cells. Immunology. 1978 Dec;35(6):981–987. [PMC free article] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. Histopathological study of protective immunity against murine salmonellosis induced by killed vaccine. Infect Immun. 1983 Jan;39(1):423–430. doi: 10.1128/iai.39.1.423-430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Hahn H. Developmental interrelationship of specific Lyt 123 and Lyt 1 cell sets in expression of antibacterial immunity to Listeria monocytogenes. Infect Immun. 1984 May;44(2):252–256. doi: 10.1128/iai.44.2.252-256.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Scovern H., Kantor F. S. Local passive transfer of delayed-type hypersensitivity in the mouse. J Immunol. 1982 Jul;129(1):25–29. [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Inoue Y., Geczy C. L., Nelson D. S. Modification of delayed-type hypersensitivity reactions to ovalbumin in cyclosporin A-treated guinea-pigs. Immunology. 1983 Feb;48(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Vingelis V., Ashman L. K., Kotlarski I. Factors affecting the ability of various strains of Enterobacteriaceae to induce tumour resistance in mice. Aust J Exp Biol Med Sci. 1980 Apr;58(2):123–131. doi: 10.1038/icb.1980.12. [DOI] [PubMed] [Google Scholar]
- Volkman A., Collins F. M. Recovery of delayed-type hypersensitivity in mice following suppressive doses of X-radiation. J Immunol. 1968 Nov;101(5):846–859. [PubMed] [Google Scholar]
- van Loveren H., Meade R., Askenase P. W. An early component of delayed-type hypersensitivity mediated by T cells and mast cells. J Exp Med. 1983 May 1;157(5):1604–1617. doi: 10.1084/jem.157.5.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]


