Abstract
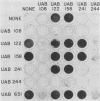
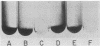
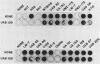
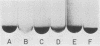
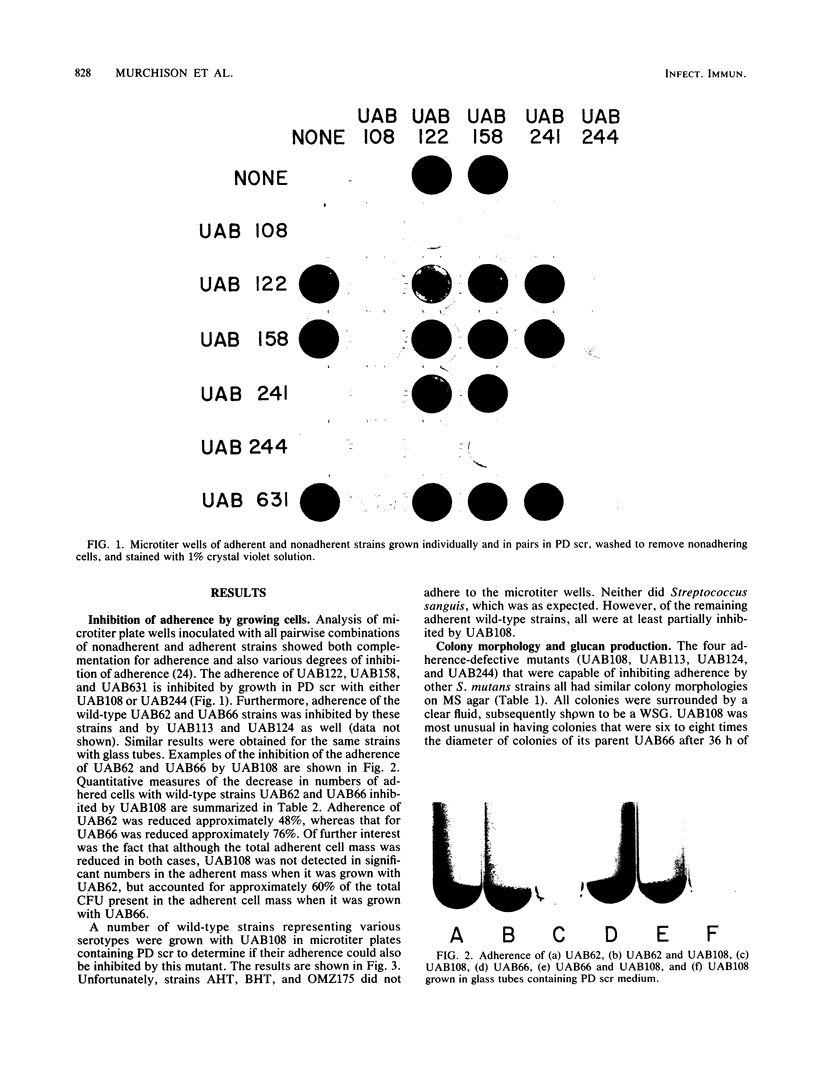
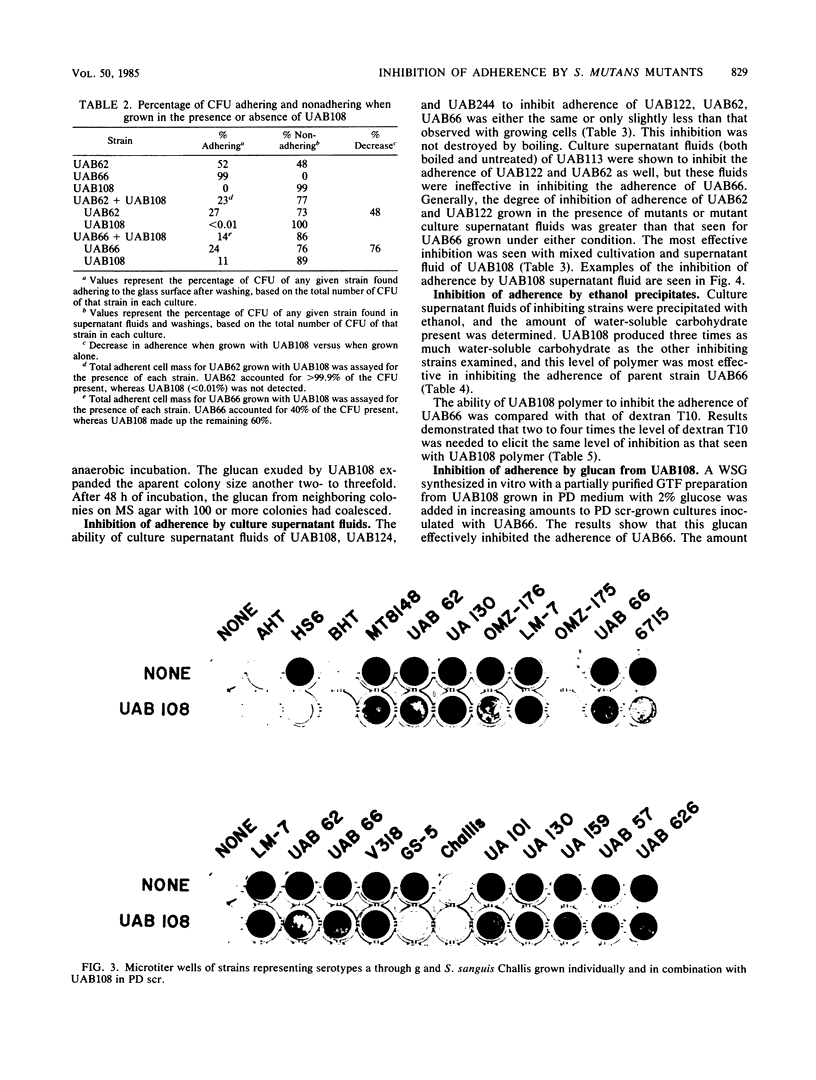
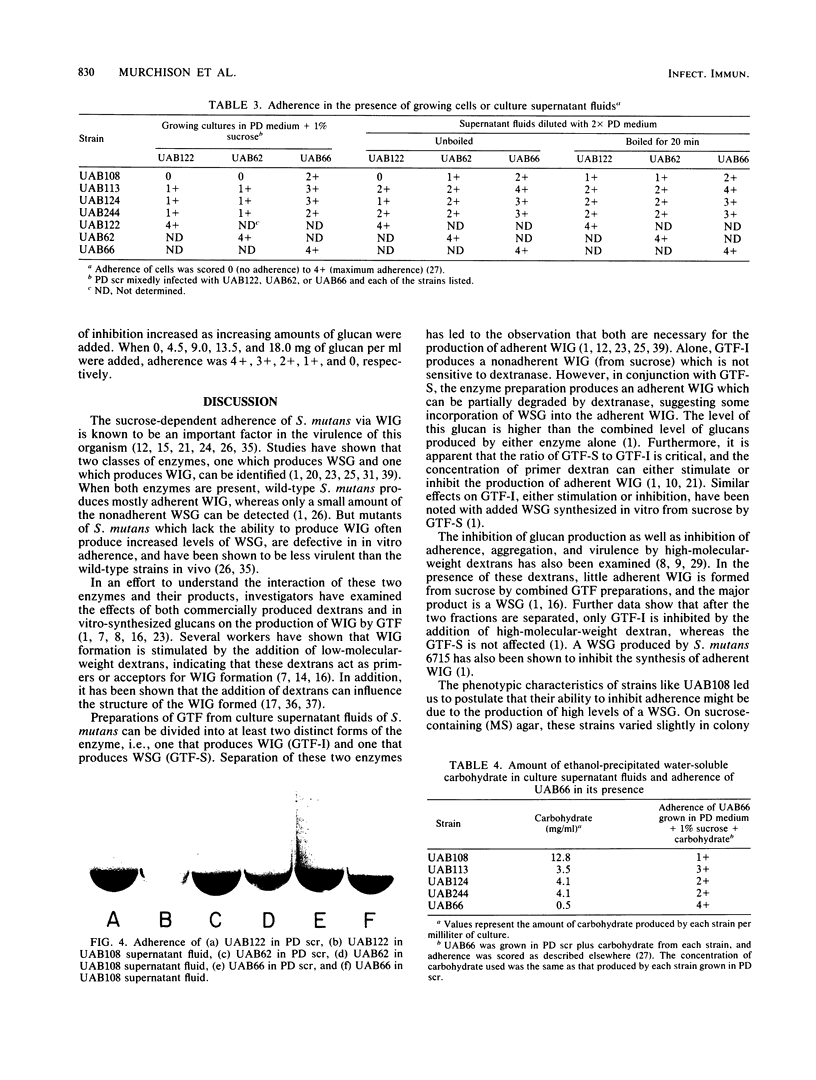
Four nonadherent mutants from Streptococcus mutans 6715 mutant UAB66 (serotype g) with similar phenotypes were shown to inhibit the adherence of adherence-proficient S. mutans serotypes c and g strains. One mutant, UAB108, was shown to inhibit adherence by wild-type strains representing serotypes a, d, and e as well. This inhibition of adherence was seen with pairs of strains grown in partially defined (PD) medium supplemented with 1% sucrose in both microtiter plates and glass tubes. The inhibiting factor was present in culture supernatant fluids of inhibiting strains grown in PD medium plus 1% sucrose and was heat stable. Ethanol precipitation of culture supernatant fluids of these strains yielded a water-soluble polymer which effectively inhibited the adherence of UAB66. This polymer, isolated from UAB108, was also shown to inhibit the adherence of UAB66 at lower concentrations than that needed to inhibit adherence with dextran T10. Partially purified glucosyltransferase, isolated from the culture supernatant fluids of glucose-grown UAB108, produced a water-soluble glucan which was shown to inhibit the adherence of UAB66 as well. The methods developed permit rapid screening for strains or mutants of strains or both that inhibit adherence or plaque formation or both by wild-type strains of S. mutans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M. D., Walker G. J. Metabolism of the polysaccharides of human dental plaque. I. Dextranase activity of streptococci, and the extracellular polysaccharides synthesized from sucrose. Caries Res. 1975;9(1):21–35. doi: 10.1159/000260139. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Guggenheim B. Dextran-induced aggregation in a mutant of Streptococcus sobrinus 6715-13. Infect Immun. 1983 Jul;41(1):264–274. doi: 10.1128/iai.41.1.264-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Hamada S., Koga T., Ooshima T. Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res. 1984 Mar;63(3):407–411. doi: 10.1177/00220345840630031001. [DOI] [PubMed] [Google Scholar]
- Hamada S., Mizuno J., Murayama Y., Ooshima Y., Masuda N. Effect of dextranase on the extracellular polysaccharide synthesis of Streptococcus mutans; chemical and scanning electron microscopy studies. Infect Immun. 1975 Dec;12(6):1415–1425. doi: 10.1128/iai.12.6.1415-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Synthesis and binding of glucosyltransferase and in vitro adherence of Streptococcus mutans grown in a synthetic medium. Arch Oral Biol. 1979;24(5):399–402. doi: 10.1016/0003-9969(79)90108-0. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M. Interaction of glucosyltransferase from Streptococcus mutans with various glucans. J Gen Microbiol. 1980 Jan;116(1):51–59. doi: 10.1099/00221287-116-1-51. [DOI] [PubMed] [Google Scholar]
- Hillman J. D. Lactate dehydrogenase mutants of Streptococcus mutans: isolation and preliminary characterization. Infect Immun. 1978 Jul;21(1):206–212. doi: 10.1128/iai.21.1.206-212.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M., Kiyono H., Shiota T., Hull R. A., Curtiss R., 3rd, Michalek S. M., McGhee J. R. Virulence of Streptococcus mutans: restoration of pathogenesis of a glucosyltransferase-defective mutant (C4). Infect Immun. 1980 Mar;27(3):915–921. doi: 10.1128/iai.27.3.915-921.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Koga T., Sato S., Hamada S. Synthesis of adherent insoluble glucan by the concerted action of the two glucosyltransferase components of Streptococcus mutans. FEBS Lett. 1982 Jun 21;143(1):101–104. doi: 10.1016/0014-5793(82)80282-2. [DOI] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Inoue M. Effects of dextranases on cell adherence, glucan-film formation and glucan synthesis by Streptococcus mutans glucosyltransferase. Arch Oral Biol. 1979;24(3):191–198. doi: 10.1016/0003-9969(79)90139-0. [DOI] [PubMed] [Google Scholar]
- Koga T., Sato S., Inoue M., Takeuchi K., Furuta T., Hamada S. Role of primers in glucan synthesis by glucosyltransferases from Streptococcus mutans strain OMZ176. J Gen Microbiol. 1983 Mar;129(3):751–754. doi: 10.1099/00221287-129-3-751. [DOI] [PubMed] [Google Scholar]
- Larrimore S., Murchison H., Shiota T., Michalek S. M., Curtiss R., 3rd In vitro and in vivo complementation of Streptococcus mutans mutants defective in adherence. Infect Immun. 1983 Nov;42(2):558–566. doi: 10.1128/iai.42.2.558-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Characterization of an anti-glucosyltransferase serum specific for insoluble glucan synthesis by Streptococcus mutans. Infect Immun. 1976 Feb;13(2):494–500. doi: 10.1128/iai.13.2.494-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants defective in adherence and aggregation. Infect Immun. 1981 Dec;34(3):1044–1055. doi: 10.1128/iai.34.3.1044-1055.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Hull S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants with altered cellular morphology or chain length. Infect Immun. 1982 Oct;38(1):282–291. doi: 10.1128/iai.38.1.282-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E., Finzen F., Sharma M. Inhibition of adherence of Streptococcus mutans to glass surfaces. Caries Res. 1977;11(3):153–159. doi: 10.1159/000260261. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Schachtele C. F. Evaluation of dextranase production by the cariogenic bacterium Streptococcus mutans. Infect Immun. 1974 Feb;9(2):467–469. doi: 10.1128/iai.9.2.467-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shiota T., Curtiss R., 3rd, Michalek S. M. Inhibition of plaque and caries formation by a glucan produced by Streptococcus mutans mutant UAB108. Infect Immun. 1985 Dec;50(3):833–843. doi: 10.1128/iai.50.3.833-843.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Brown R. A., Taylor C. Activity of Streptococcus mutans alpha-D-glucosyltransferases released under various growth conditions. J Dent Res. 1984 Mar;63(3):397–400. doi: 10.1177/00220345840630030801. [DOI] [PubMed] [Google Scholar]