Abstract
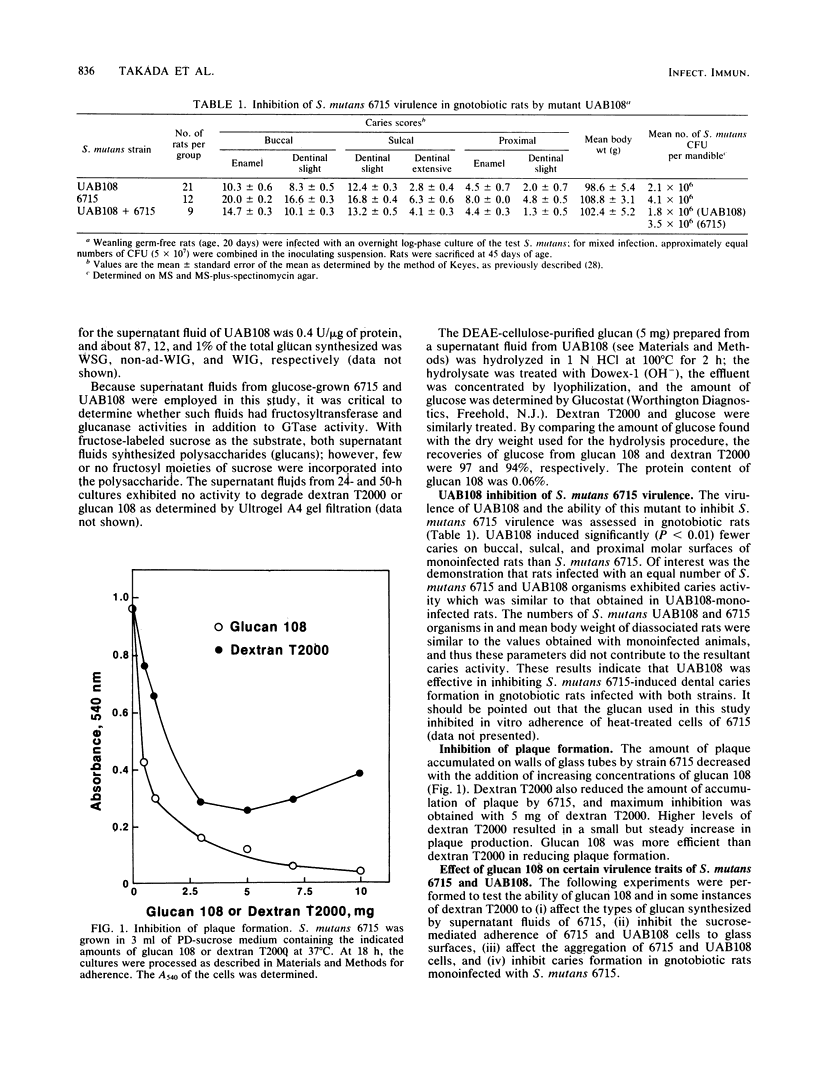
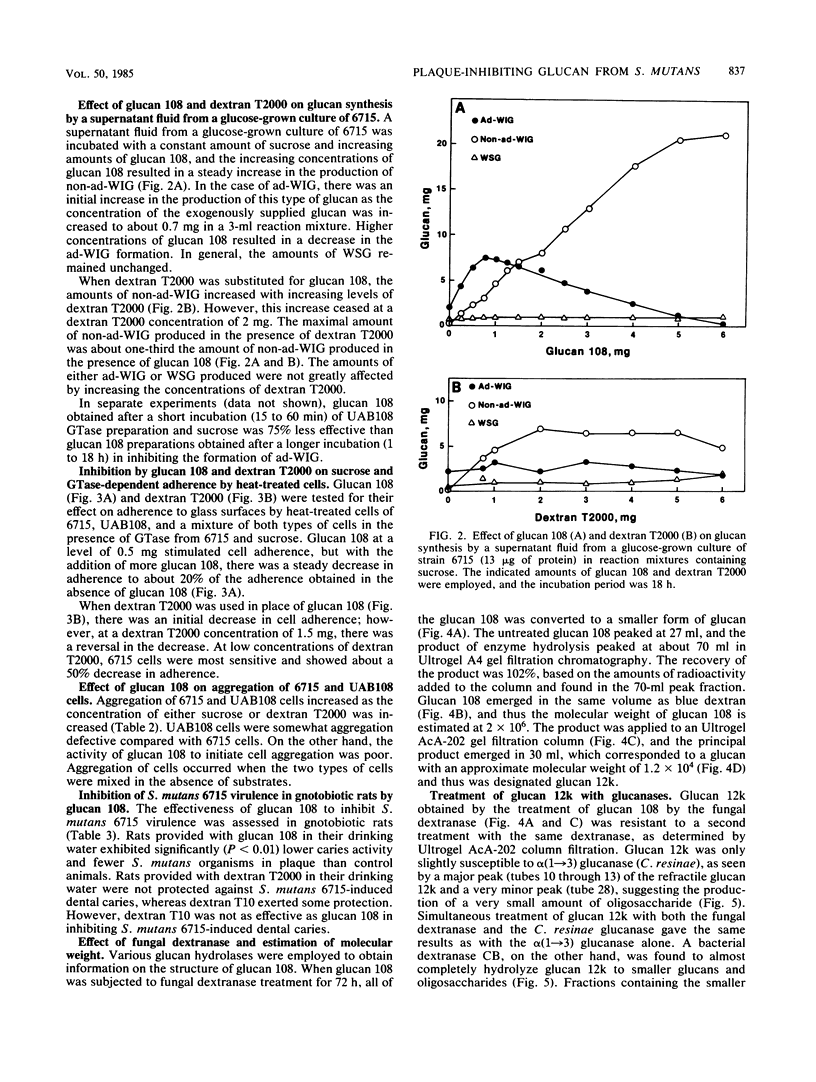
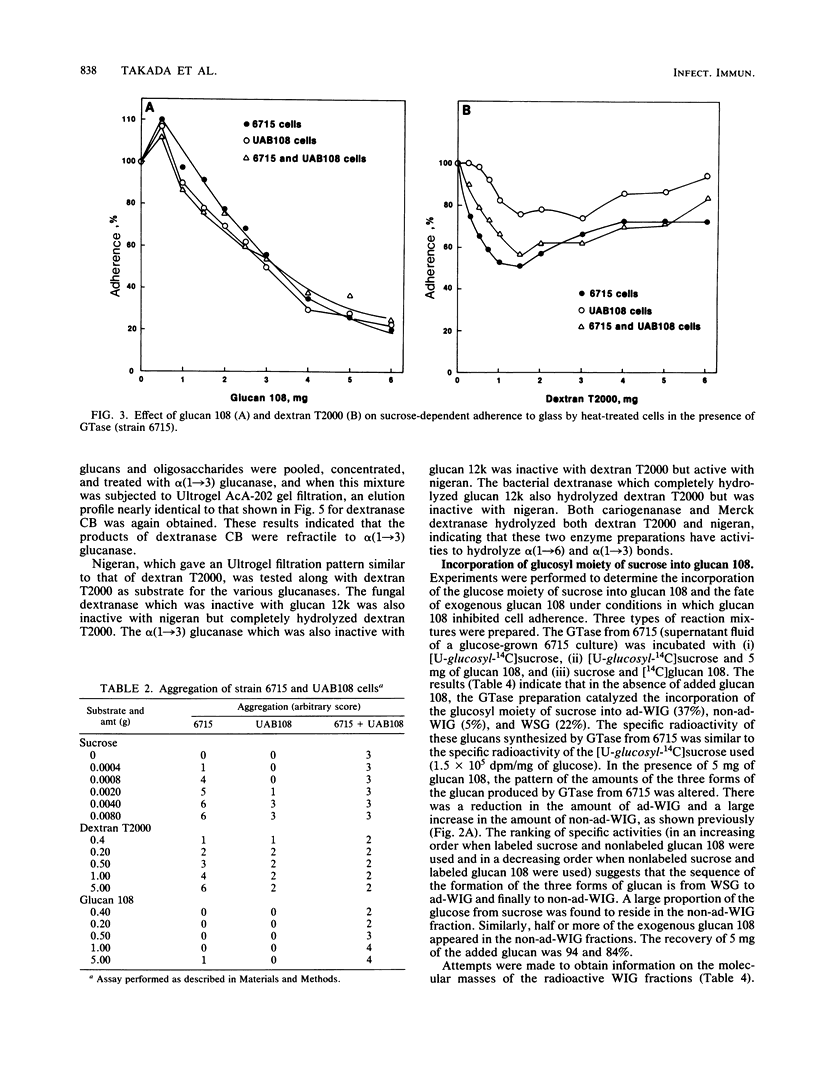
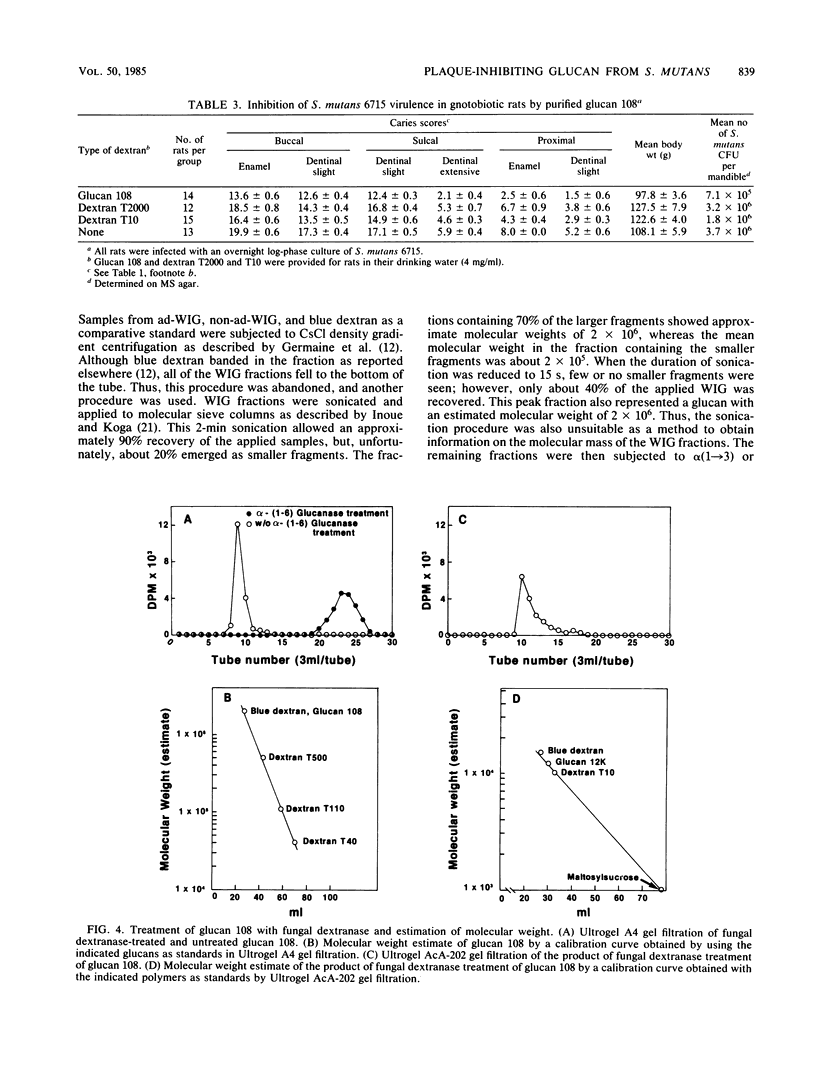
A mutant (UAB108) derived from Streptococcus mutans UAB66, a spectinomycin-resistant (Spcr) isolate of strain 6715, inhibited plaque formation when grown with strain 6715 in a sucrose medium and also inhibited caries formation in gnotobiotic rats infected with both strain UAB108 and 6715. A substance obtained from UAB108 culture supernatant fluid after ethanol precipitation and DEAE-cellulose treatment, designated glucan 108, inhibited S. mutans 6715 virulence and was shown to be a water-soluble glucan. In the presence of sucrose and increasing concentrations of glucan 108, the activity of a glucosyltransferase (GTase) preparation from S. mutans 6715 to synthesize adhesive water-insoluble glucan (ad-WIG) was inhibited, and the activity to synthesize non-ad-WIG was stimulated. Glucan 108 similarly inhibited sucrose-dependent adherence of heat-treated cells, was a poor inducer of cell aggregation, and inhibited S. mutans 6715-induced dental caries in gnotobiotic rats. In the presence of GTase, glucan 108, and sucrose, the glucose moiety of sucrose was found to be incorporated into glucan 108, and most of this glucose-incorporated glucan 108 was found in the non-ad-WIG fraction. The mode of inhibition of plaque formation by S. mutans 6715 appears to involve a shift from ad-WIG to non-ad-WIG formation. The water-soluble glucan 108 was found to have an approximate molecular weight of 2 X 10(6) and was hydrolyzed by fungal dextranase to yield glucans with an average molecular weight of about 1.2 X 10(4). This glucan (designated glucan 12k) was further hydrolyzed by bacterial dextranase to yield smaller glucans and oligosaccharides, but was refractile to alpha (1----3) glucanase. These results suggest that glucan 108 is a branched alpha (1----6) glucan, and it is proposed that UAB108 is defective in its ability to polymerize glucan 12k with alpha (1----3)-linked glucosyl residues.
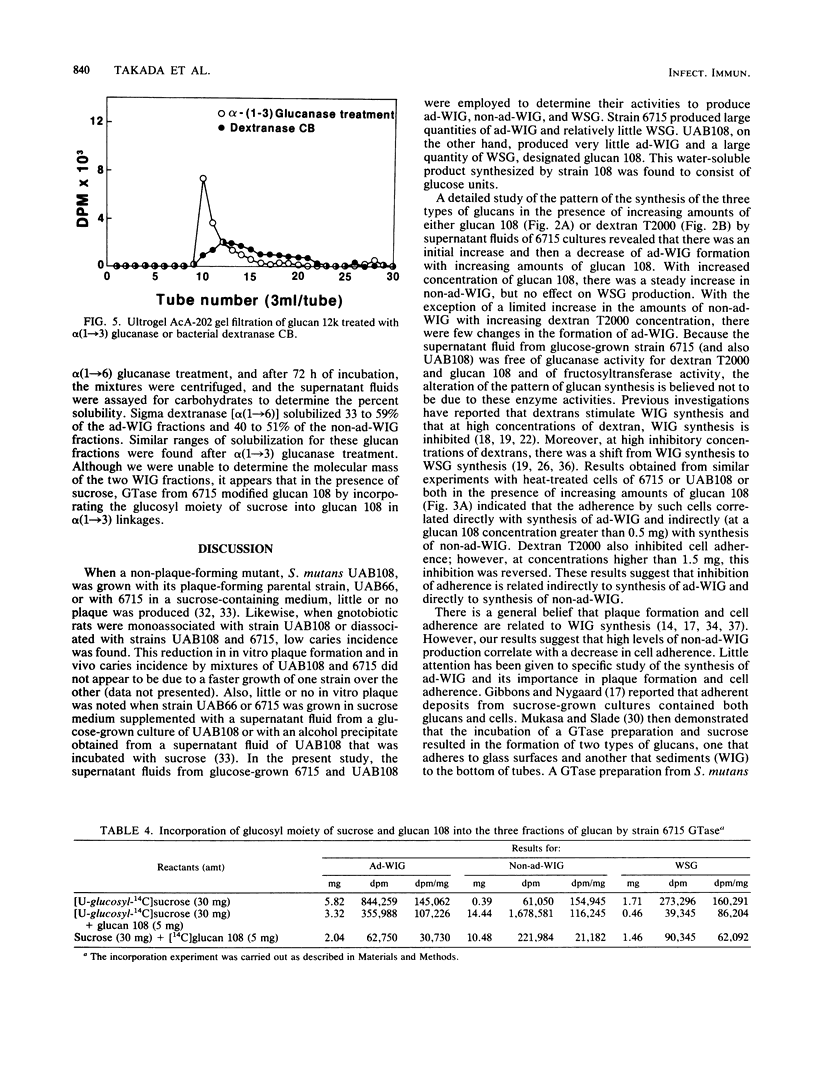
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Beaman A. J., Wittenberger C. L. Purification, resolution, and interaction of the glucosyltransferases of Streptococcus mutans 6715. Infect Immun. 1977 Oct;18(1):237–246. doi: 10.1128/iai.18.1.237-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Guggenheim B. Dextran-induced aggregation in a mutant of Streptococcus sobrinus 6715-13. Infect Immun. 1983 Jul;41(1):264–274. doi: 10.1128/iai.41.1.264-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Birked D., Granath K. Analyses of glucans from cariogenic and mutant Streptococcus mutans. Infect Immun. 1978 Jul;21(1):17–27. doi: 10.1128/iai.21.1.17-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Moriyama T., Miyake Y., Mizutani K., Tanaka O. Purification and properties of glucosyltransferase responsible for water-insoluble glucan synthesis from Streptococcus mutans. Infect Immun. 1982 Jul;37(1):1–9. doi: 10.1128/iai.37.1.1-9.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Takada K., Motoda R., Ikeda T. Independence of water-insoluble glucan synthesis and adherence of Streptococcus mutans to smooth surfaces. FEBS Lett. 1982 Nov 29;149(2):299–303. doi: 10.1016/0014-5793(82)81121-6. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M. Interaction of glucosyltransferase from Streptococcus mutans with various glucans. J Gen Microbiol. 1980 Jan;116(1):51–59. doi: 10.1099/00221287-116-1-51. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Shiota T., McGhee J. R., Otake S., Michalek S. M., Ochiai K., Hirasawa M., Sugimoto K. Virulence of Streptococcus mutans: comparison of the effects of a coupling sugar and sucrose on certain metabolic activities and cariogenicity. Infect Immun. 1978 Feb;19(2):477–480. doi: 10.1128/iai.19.2.477-480.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Koga T. Fractionation and properties of glucans produced by Streptococcus mutans. Infect Immun. 1979 Sep;25(3):922–931. doi: 10.1128/iai.25.3.922-931.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Sato S., Inoue M., Takeuchi K., Furuta T., Hamada S. Role of primers in glucan synthesis by glucosyltransferases from Streptococcus mutans strain OMZ176. J Gen Microbiol. 1983 Mar;129(3):751–754. doi: 10.1099/00221287-129-3-751. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrimore S., Murchison H., Shiota T., Michalek S. M., Curtiss R., 3rd In vitro and in vivo complementation of Streptococcus mutans mutants defective in adherence. Infect Immun. 1983 Nov;42(2):558–566. doi: 10.1128/iai.42.2.558-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Relationship between cell-bound dextransucrase and the agglutination of Streptococcus mutans. Infect Immun. 1975 Sep;12(3):512–520. doi: 10.1128/iai.12.3.512-520.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Navia J. M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975 Jul;12(1):69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Shiota T., Devenyns D. Low sucrose levels promote extensive Streptococcus mutans-induced dental caries. Infect Immun. 1977 May;16(2):712–714. doi: 10.1128/iai.16.2.712-714.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Curtiss R., 3rd In vitro inhibition of adherence of Streptococcus mutans strains by nonadherent mutants of S. mutans 6715. Infect Immun. 1985 Dec;50(3):826–832. doi: 10.1128/iai.50.3.826-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants defective in adherence and aggregation. Infect Immun. 1981 Dec;34(3):1044–1055. doi: 10.1128/iai.34.3.1044-1055.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- Newbrun E., Finzen F., Sharma M. Inhibition of adherence of Streptococcus mutans to glass surfaces. Caries Res. 1977;11(3):153–159. doi: 10.1159/000260261. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Corrigan A. J. The mechanism of dextransucrase action. Activation of dextransucrase from Streptococcus mutans OMZ 176 by dextran and modified dextran and the nonexistence of the primar requirement for the synthesis of dextran. Arch Biochem Biophys. 1977 Oct;183(2):726–731. doi: 10.1016/0003-9861(77)90406-4. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Critchley P. The extracellular polysaccharide produced from sucrose by a cariogenic streptococcus. Arch Oral Biol. 1966 Oct;11(10):1039–1042. doi: 10.1016/0003-9969(66)90204-4. [DOI] [PubMed] [Google Scholar]
- Wu-Yuan C. D., Tai S., Slade H. D. Dextran/glucan binding by Streptococcus mutans: the role of molecular size and binding site in agglutination. Adv Exp Med Biol. 1978;107:737–748. doi: 10.1007/978-1-4684-3369-2_83. [DOI] [PubMed] [Google Scholar]


