Abstract
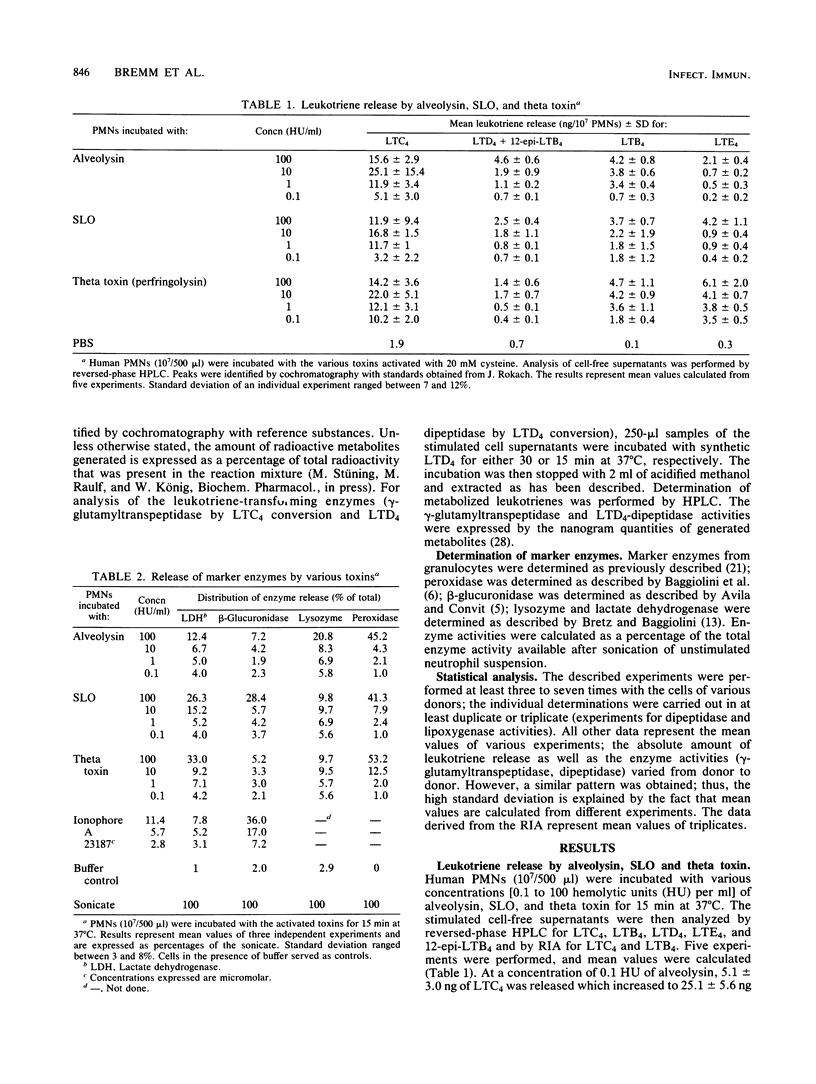
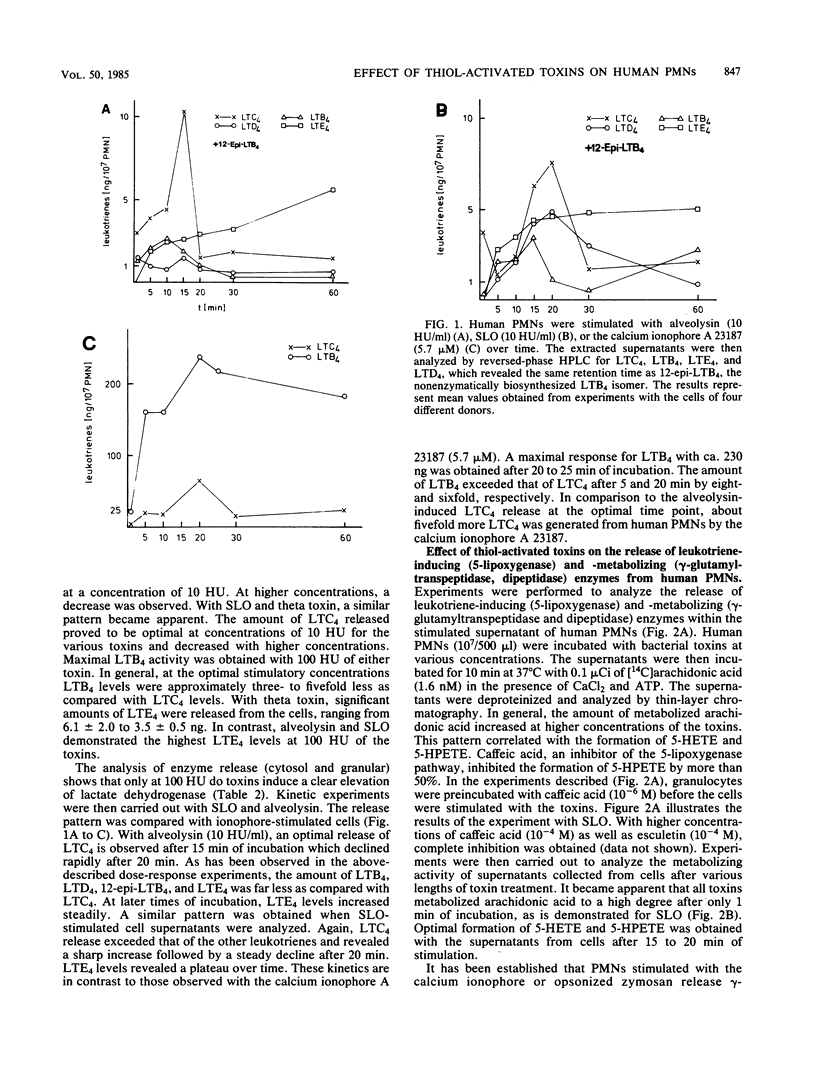
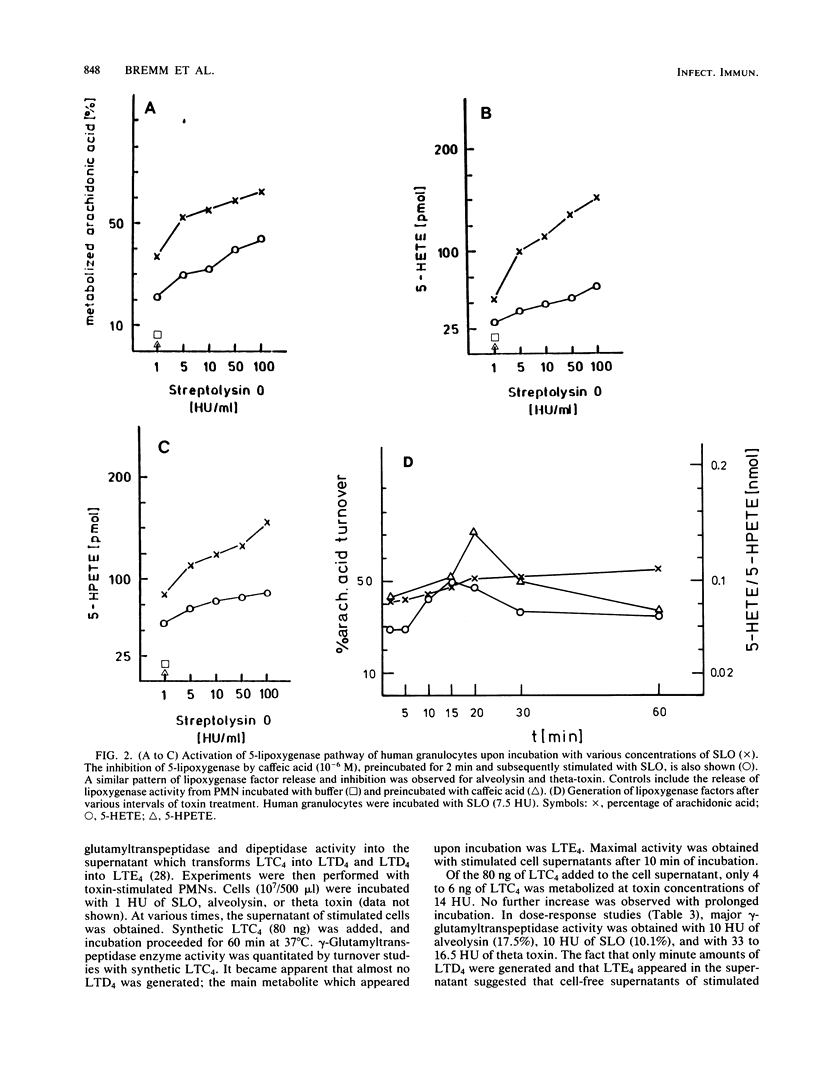
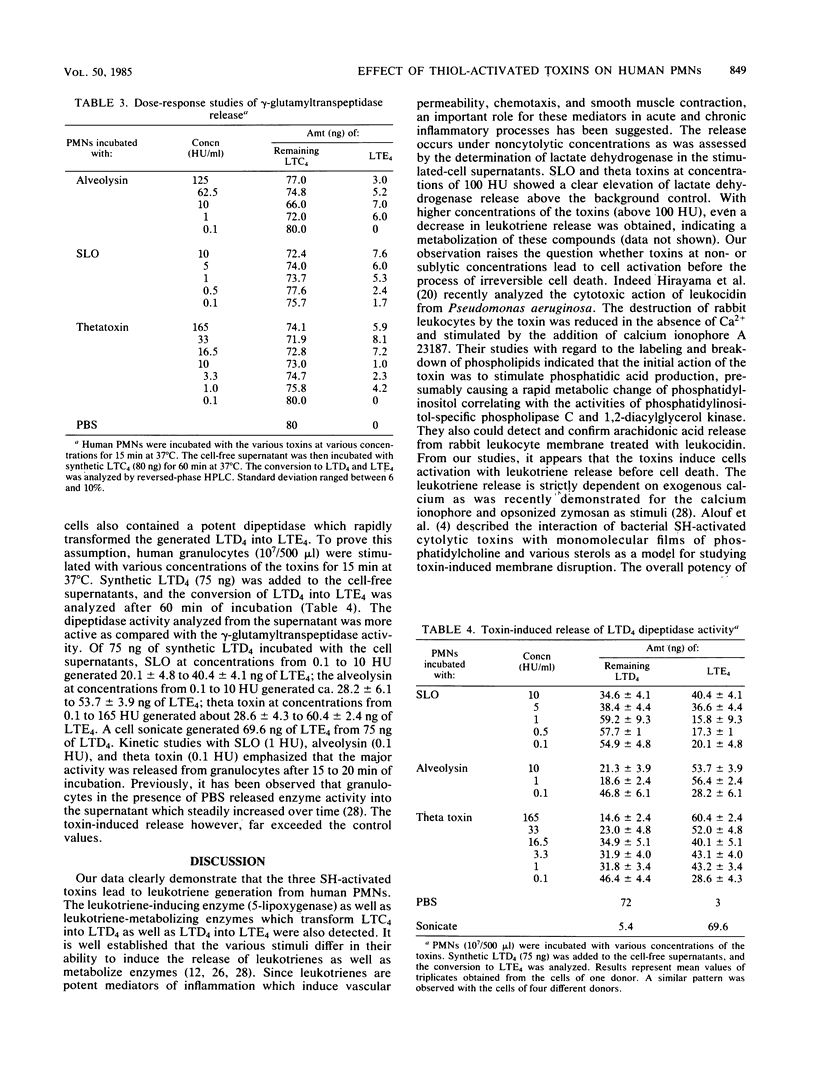
The generation of leukotrienes (LTC4, LTD4, LTE4, and LTB4; 12-epi-LTB4 isomer) from human granulocytes by thiol-activated toxins (streptolysin O, alveolysin from Bacillus alvei, and theta toxin from Clostridium perfringens) is described. The release occurs under noncytolytic conditions. Although LTB4 is the major component after calcium ionophore stimulation, more LTC4 as compared with LTB4 is released with the toxins. The 5-lipoxygenase pathway of toxin-mediated activation can effectively be inhibited by caffeic acid, a lipoxygenase inhibitor. The toxins also induce the release of leukotriene-metabolizing enzymes such as gamma-glutamyltranspeptidase, which transfers LTC4 into LTD4, and dipeptidase, which metabolizes LTD4, into LTE4. Dipeptidase activity is more pronounced than the gamma-glutamyltranspeptidase activity but still does not reach the levels obtained when cells were triggered with opsonized zymosan.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aehringhaus U., Wölbling R. H., König W., Patrono C., Peskar B. M., Peskar B. A. Release of leukotriene C4 from human polymorphonuclear leucocytes as determined by radioimmunoassay. FEBS Lett. 1982 Sep 6;146(1):111–114. doi: 10.1016/0014-5793(82)80715-1. [DOI] [PubMed] [Google Scholar]
- Alouf J. E., Geoffroy C., Pattus F., Verger R. Surface properties of bacterial sulfhydryl-activated cytolytic toxins. Interaction with monomolecular films of phosphatidylcholine and various sterols. Eur J Biochem. 1984 May 15;141(1):205–210. doi: 10.1111/j.1432-1033.1984.tb08176.x. [DOI] [PubMed] [Google Scholar]
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Studies on human polymorphonuclear leukocyte enzymes. I. Assay of acid hydrolases and other enzymes. Biochim Biophys Acta. 1973 Feb 15;293(2):397–408. doi: 10.1016/0005-2744(73)90347-1. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Fruteau de Laclos B., Rabinovitch H., Picard S., Braquet P., Hébert J., Laviolette M. Eosinophil-rich human polymorphonuclear leukocyte preparations characteristically release leukotriene C4 on ionophore A23187 challenge. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):310–315. doi: 10.1016/0091-6749(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Bray M. A., Cunningham F. M., Ford-Hutchinson A. W., Smith M. J. Leukotriene B4: a mediator of vascular permeability. Br J Pharmacol. 1981 Mar;72(3):483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom H. J., Alouf J. E., König W., Spur B., Crea A., Peters W. Generation of leukotrienes from human granulocytes by alveolysin from Bacillus alvei. Infect Immun. 1984 Apr;44(1):188–193. doi: 10.1128/iai.44.1.188-193.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom J., Raulf M., Stüning M., Spur B., Crea A., Bremm K. D., König W. Subcellular localization of enzymes involved in leukotriene formation within human polymorphonuclear granulocytes. Immunology. 1984 Mar;51(3):571–583. [PMC free article] [PubMed] [Google Scholar]
- Freer J. H. Cytolytic toxins and surface activity. Toxicon. 1982;20(1):217–221. doi: 10.1016/0041-0101(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Alouf J. E. Interaction of alveolysin A sulfhydryl-activated bacterial cytolytic toxin with thiol group reagents and cholesterol. Toxicon. 1982;20(1):239–241. doi: 10.1016/0041-0101(82)90208-2. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Alouf J. E. Selective purification by thiol-disulfide interchange chromatography of alveolysin, a sulfhydryl-activated toxin of Bacillus alvei. Toxin properties and interaction with cholesterol and liposomes. J Biol Chem. 1983 Aug 25;258(16):9968–9972. [PubMed] [Google Scholar]
- Hirayama T., Kato I. Mode of cytotoxic action of pseudomonal leukocidin on phosphatidylinositol metabolism and activation of lysosomal enzyme in rabbit leukocytes. Infect Immun. 1984 Jan;43(1):21–27. doi: 10.1128/iai.43.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshihara Y., Neichi T., Murota S., Lao A., Fujimoto Y., Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta. 1984 Jan 17;792(1):92–97. [PubMed] [Google Scholar]
- Köller M., Schönfeld W., Knöller J., Bremm K. D., König W., Spur B., Crea A., Peters W. The metabolism of leukotrienes in blood plasma studied by high-performance liquid chromatography. Biochim Biophys Acta. 1985 Jan 9;833(1):128–134. doi: 10.1016/0005-2760(85)90260-7. [DOI] [PubMed] [Google Scholar]
- König W., Frickhofen N., Tesch H. Generation and secretion of eosinophilotactic activity from human polymorphonuclear neutrophils by various mechanisms of cell activation. Immunology. 1979 Apr;36(4):733–742. [PMC free article] [PubMed] [Google Scholar]
- Lazdunski C., Cavard D. Colicins: a minireview. Toxicon. 1982;20(1):223–228. doi: 10.1016/0041-0101(82)90205-7. [DOI] [PubMed] [Google Scholar]
- Möllby R., Thelestam M., Geoffroy C., Alouf J. E. Two different modes of membrane damaging action by bacterial thiol-activated haemolysins. Toxicon. 1982;20(1):229–232. doi: 10.1016/0041-0101(82)90206-9. [DOI] [PubMed] [Google Scholar]
- Neichi T., Koshihara Y., Murota S. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim Biophys Acta. 1983 Aug 29;753(1):130–132. [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Raulf M., Stüning M., König W. Metabolism of leukotrienes by L-gamma-glutamyl-transpeptidase and dipeptidase from human polymorphonuclear granulocytes. Immunology. 1985 May;55(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Determination of toxin-induced leakage of different-size nucleotides through the plasma membrane of human diploid fibroblasts. Infect Immun. 1975 Apr;11(4):640–648. doi: 10.1128/iai.11.4.640-648.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Interaction of streptolysin O from Streptococcus pyogenes and theta-toxin from Clostridium perfringens with human fibroblasts. Infect Immun. 1980 Sep;29(3):863–872. doi: 10.1128/iai.29.3.863-872.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


