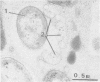
Abstract
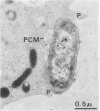
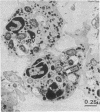
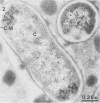
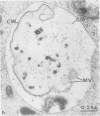
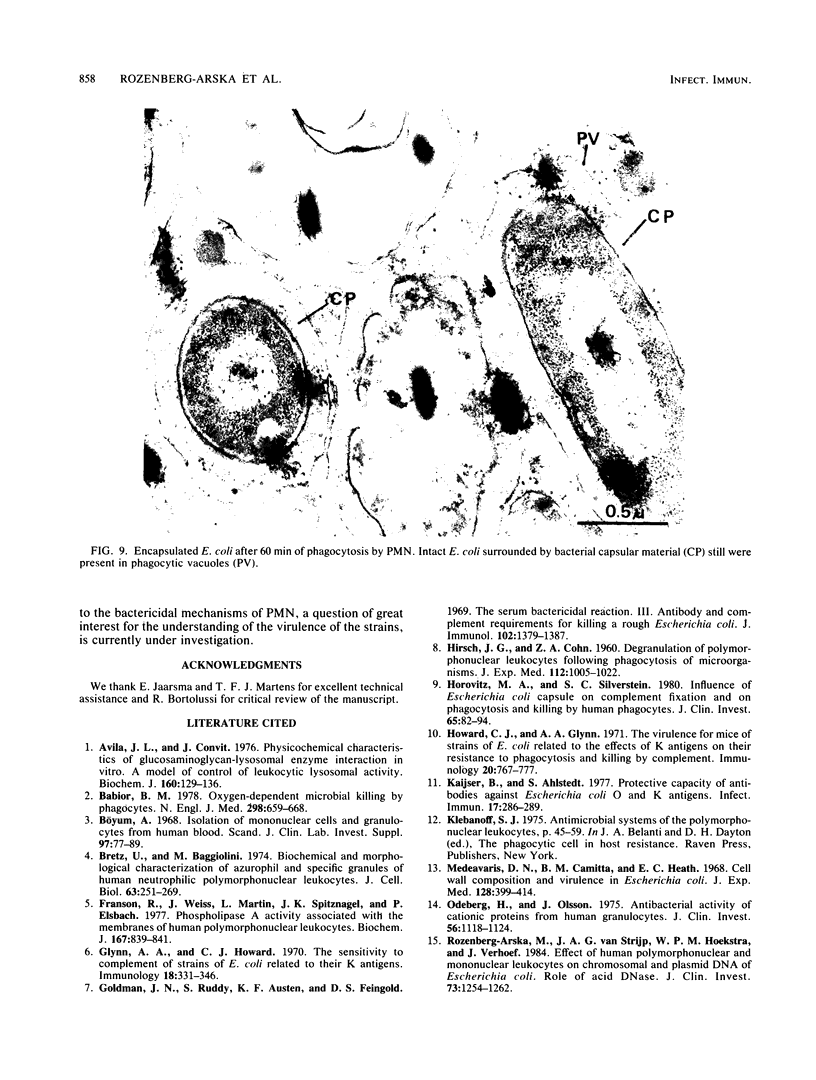
The fate of Escherichia coli strains within the polymorphonuclear leukocytes was studied by determining the killing of bacteria, measuring the release of degradation products, and examining the phagocytic bacteria by electron microscopy. When sufficiently opsonized, both unencapsulated and encapsulated E. coli strains were rapidly phagocytized by polymorphonuclear leukocytes. Once phagocytized, the two unencapsulated E. coli strains (K-12 and O111) were rapidly killed (99% of the bacteria were killed during the first 5 min of phagocytosis) and extensively degraded (about 40% of the radiolabeled material was released from bacteria after 15 min of phagocytosis). Electron micrographs taken after 15 min of phagocytosis revealed extensive structural changes in most of the internalized bacteria. In contrast to the rapid killing and extensive breakdown of these strains, encapsulated E. coli O78:K80 was more resistant to killing and withstood degradation by polymorphonuclear leukocytes (only 5% of the radioactivity was released from the radiolabeled bacteria after 1 h of phagocytosis). Electron micrographs of thin sections taken after 1 h of phagocytosis revealed virtually no structural changes. Most of the internalized bacteria were still surrounded by thick capsular material.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Convit J. Physicochemical characteristics of the glycosaminoglycan-lysosomal enzyme interaction in vitro. A model of control of leucocytic lysosomal activity. Biochem J. 1976 Nov 15;160(2):129–136. doi: 10.1042/bj1600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Franson R., Weiss J., Martin L., Spitznagel J. K., Elsbach P. Phospholipase A activity associated with membranes of human polymorphonuclear leucocytes. Biochem J. 1977 Dec 1;167(3):839–841. doi: 10.1042/bj1670839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Goldman J. N., Ruddy S., Austen K. F., Feingold D. S. The serum bactericidal reaction. 3. Antibody and complement requirements for killing a rough Escherichia coli. J Immunol. 1969 Jun;102(6):1379–1387. [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Kaijser B., Ahlstedt S. Protective capacity of antibodies against Escherichia coli and K antigens. Infect Immun. 1977 Aug;17(2):286–289. doi: 10.1128/iai.17.2.286-289.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., van Strijp J. A., Hoekstra W. P., Verhoef J. Effect of human polymorphonuclear and mononuclear leukocytes on chromosomal and plasmid DNA of Escherichia coli. Role of acid DNase. J Clin Invest. 1984 May;73(5):1254–1262. doi: 10.1172/JCI111327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Recognition and response in mononuclear and granular phagocytes. Clin Exp Immunol. 1976 Sep;25(3):355–366. [PMC free article] [PubMed] [Google Scholar]