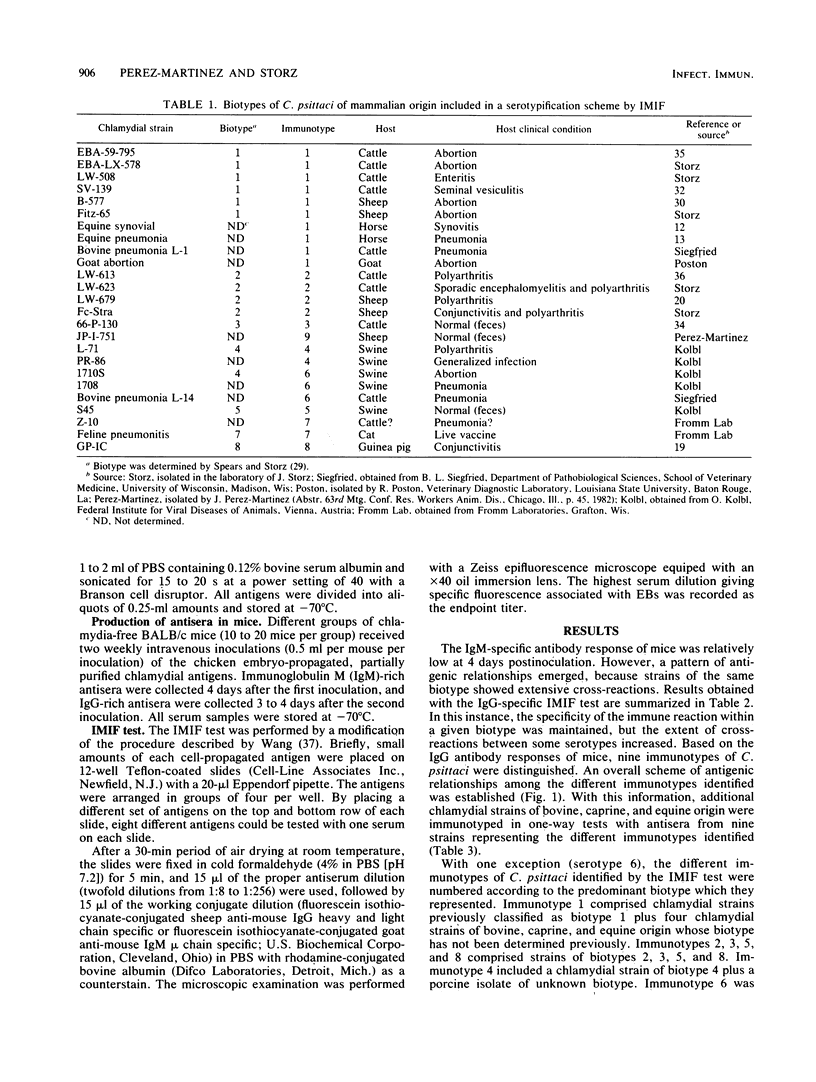
Abstract
A group of twenty-five isolates of Chlamydia psittaci representing at least seven different biotypes of bovine, ovine, caprine, equine, feline, porcine, and guinea pig origin were immunotyped by an indirect microimmunofluorescence test. Different groups of chlamydia-free BALB/c mice received two weekly intravenous inoculations with chicken embryo-propagated, partially purified elementary bodies of each strain. Antisera for immunotyping were collected 4 days after the first inoculation and 3 to 4 days after the second inoculation and tested for antichlamydial immunoglobulin M and immunoglobulin G antibodies by the indirect microimmunofluorescence test with cell culture-propagated, partially purified homologous and heterologous antigens. Nine immunotypes of C. psittaci were distinguished. The correlation between immunotypes and biotypes was close, and a pattern of either disease or host specificity could be associated with each immunotype. Most immunotypes identified induced cross-reacting antibodies against each other, but no significant cross-reactions were observed with elementary bodies of the mouse pneumonitis strain of C. trachomatis. Findings from this study should provide the necessary background for the rational selection of prototype strains of C. psittaci for further antigenic analysis at the molecular level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Judd R. C. Structural analysis of chlamydial major outer membrane proteins. Infect Immun. 1982 Dec;38(3):960–968. doi: 10.1128/iai.38.3.960-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. II. A trachoma-LGV-specific antigen. J Immunol. 1975 Oct;115(4):969–975. [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eb F., Orfila J. Serotyping of Chlamydia psittaci by the micro-immunofluorescence test: isolates of ovine origin. Infect Immun. 1982 Sep;37(3):1289–1291. doi: 10.1128/iai.37.3.1289-1291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER C. E., BERMAN D. T. TYPE-SPECIFIC ANTIGENS IN THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM GROUP OF ORGANISMS. J Bacteriol. 1965 Apr;89:943–948. doi: 10.1128/jb.89.4.943-948.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Johnson M. C., Grimes J. E. Resistance of wild birds to infection by Chlamydia psittaci of mammalian origin. J Infect Dis. 1983 Jan;147(1):162–162. doi: 10.1093/infdis/147.1.162. [DOI] [PubMed] [Google Scholar]
- Lee C. K. Interaction between a trachoma strain of Chlamydia trachomatis and mouse fibroblasts (McCoy cells) in the absence of centrifugation. Infect Immun. 1981 Feb;31(2):584–591. doi: 10.1128/iai.31.2.584-591.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- McChesney A. E., Becerra V., England J. J. Chlamydial polyarthritis in a foal. J Am Vet Med Assoc. 1974 Aug 1;165(3):259–261. [PubMed] [Google Scholar]
- McChesney S. L., England J. J., McChesney A. E. Chlamydia psittaci induced pneumonia in a horse. Cornell Vet. 1982 Jan;72(1):92–97. [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. L., Storz J. Observations on sheep with polyarthritis produced by an agent of the psittacosis-lymphogranuloma venereum-trachoma group. Arthritis Rheum. 1967 Feb;10(1):1–12. doi: 10.1002/art.1780100102. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Reed D. E., Lincoln S. D., Kwapian R. P., Chow T. L., Whiteman C. E. Comparison of antigenic structure and pathogenicity of bovine intestinal Chlamydia isolate with an agent of epizootic bovine abortion. Am J Vet Res. 1975 Aug;36(08):1141–1143. [PubMed] [Google Scholar]
- Sayed H., Fung K., Wilt J. C. Differences in physicochemical and antigenic properties of chlamydial strains. Can J Microbiol. 1976 Jul;22(7):937–941. doi: 10.1139/m76-135. [DOI] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Chlamydia psittaci: growth characteristics and enumeration of serotypes 1 and 2 in cultured cells. J Infect Dis. 1979 Dec;140(6):959–967. doi: 10.1093/infdis/140.6.959. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Storz J., Carroll E. J., Ball L., Faulkner L. C. Isolation of a psittacosis agent (Chlamydia) from semen and epididymis of bulls with seminal vesiculitis syndrome. Am J Vet Res. 1968 Mar;29(3):549–555. [PubMed] [Google Scholar]
- Storz J., Marriott M. E., Winterer B. I. Detection and separation of simultaneous Enterovirus and intestinal Chlamydia infection of calves. Zentralbl Bakteriol Orig. 1969 May;210(1):75–81. [PubMed] [Google Scholar]
- Storz J. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J Comp Pathol. 1966 Oct;76(4):351–362. doi: 10.1016/0021-9975(66)90055-7. [DOI] [PubMed] [Google Scholar]
- Storz J., Smart R. A., Marriott M. E., Davis R. V. Polyarthritis of calves: isolation of psittacosis agents from affected joints. Am J Vet Res. 1966 May;27(118):633–641. [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. Formalinized Chlamydia trachomatis organisms as antigen in the micro-immunofluorescence test. J Clin Microbiol. 1979 Aug;10(2):259–261. doi: 10.1128/jcm.10.2.259-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R. The differentiation of some psittacosis-lymphogranuloma venereum gropu agents isolated from sheep and cattle. J Infect Dis. 1966 Jun;116(3):319–323. doi: 10.1093/infdis/116.3.319. [DOI] [PubMed] [Google Scholar]


