Abstract
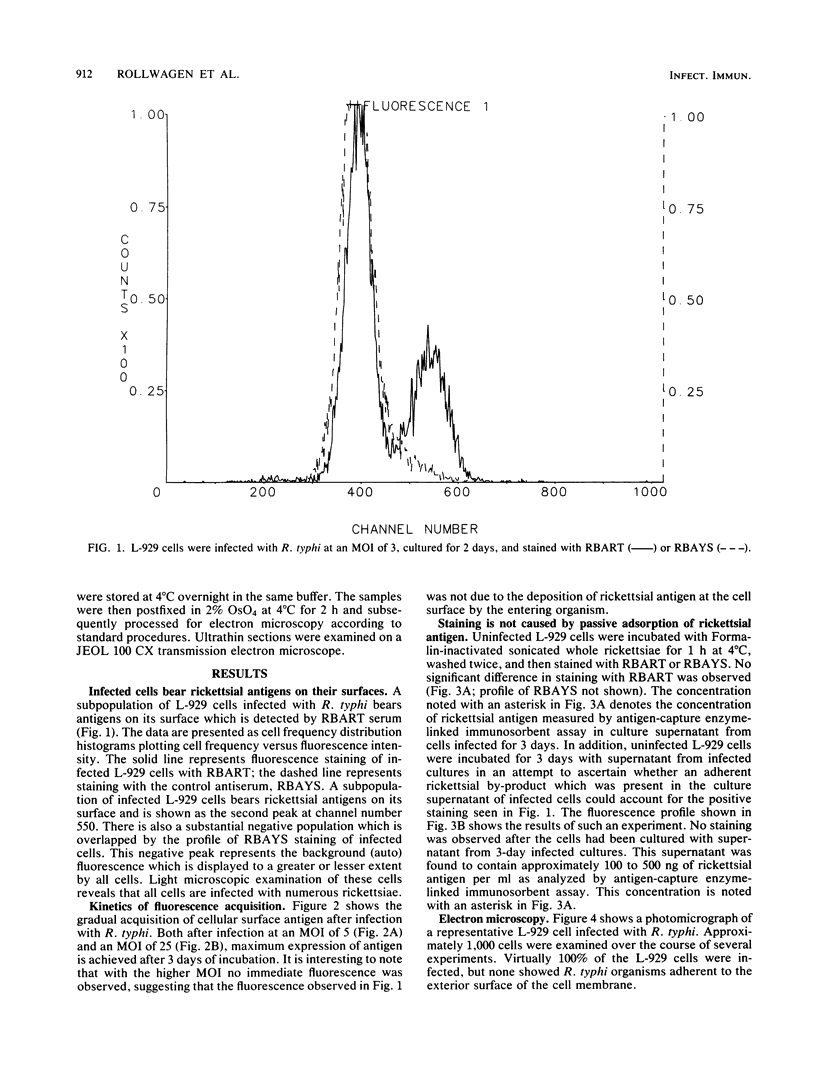
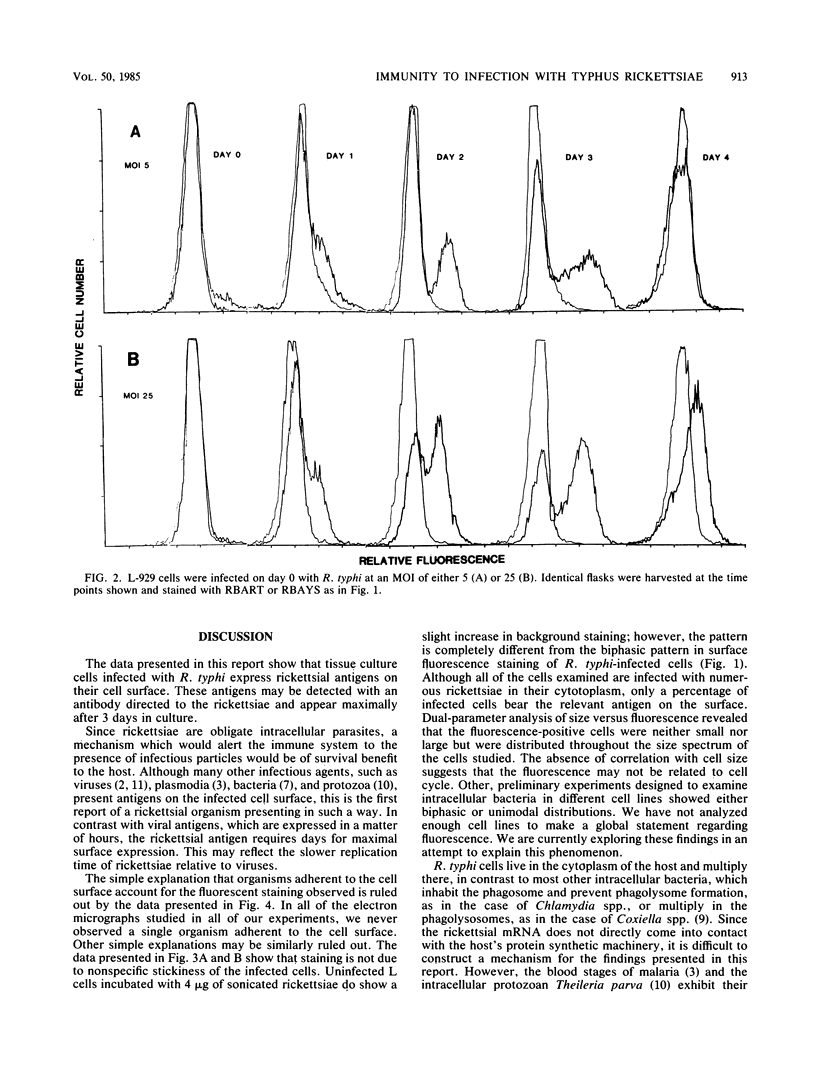
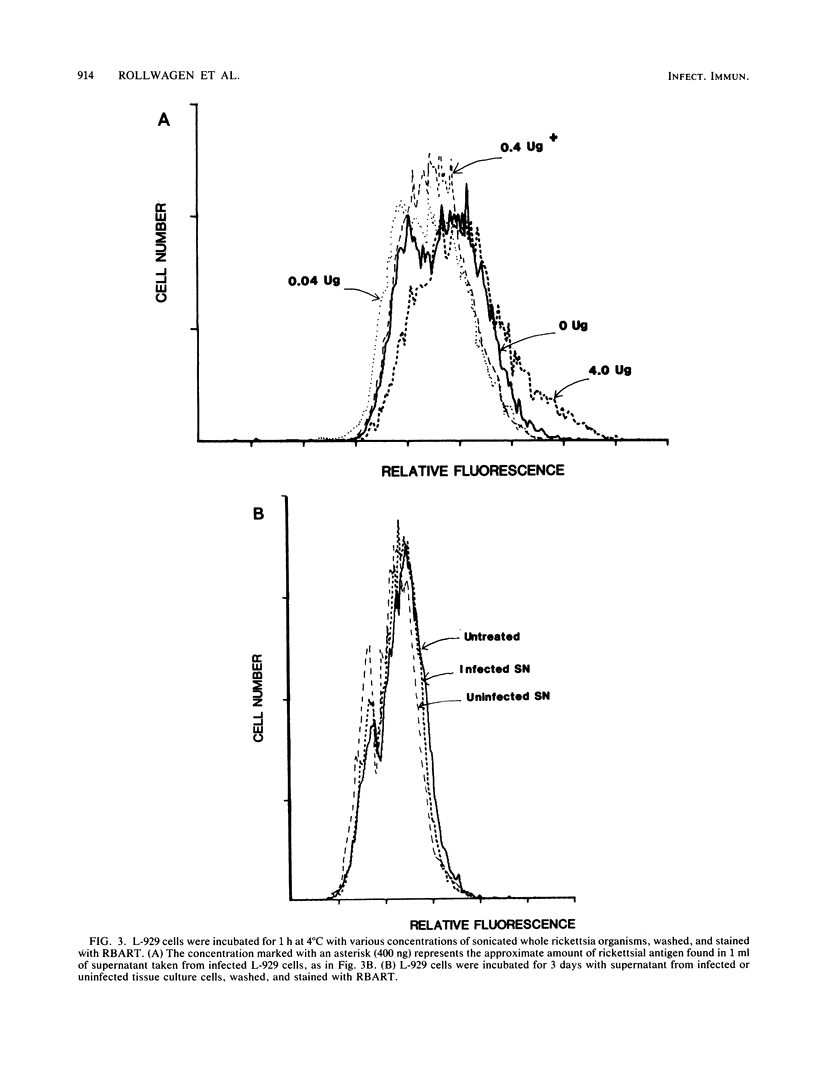
As with any immune response to infectious organisms, both antibody and T cell-mediated immune responses to infection with Rickettsia typhi require the appropriate presentation of rickettsial antigens to immunocompetent cells. Considering the obligate intracellular nature of rickettsiae, the exact mechanisms by which lymphocytes and macrophages encounter and respond to rickettsial antigens may depend on certain aspects of pathogenesis and on the availability of organisms or their antigens to cells of the immune system. One potential mode of rickettsial antigen presentation, not previously identified, is the appearance in vitro of rickettsial antigens on the cell membrane of R. typhi-infected L-929 fibroblasts. Polyvalent fluoresceinated rabbit antisera directed against whole R. typhi cells used in flow cytometric analysis of infected fibroblasts showed an increasing presence of R. typhi antigen on the host cell membrane 1 to 3 days postinfection. The significance of this finding in the pathophysiology of rickettsia-host interactions and the generation of cytotoxic T cell-mediated immunity and antibody immunity is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Philip R. N., Casper E., Todd W. J., Mann R. E., Johnston M. R., Nauck C. J. Biological properties of rabbit antibodies to a surface antigen of Rickettsia rickettsii. Infect Immun. 1983 Apr;40(1):292–298. doi: 10.1128/iai.40.1.292-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Murine leukemia virus proteins expressed on the surface of infected cells in culture. J Virol. 1980 Mar;33(3):936–944. doi: 10.1128/jvi.33.3.936-944.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Kokorin I. N., Kabanova E. A., Shirokova E. M., Abrosimova G. E., Rybkina N. N., Pushkareva V. i. Role of T lymphocytes in Rickettsia conorii infection. Acta Virol. 1982 Jan;26(1-2):91–97. [PubMed] [Google Scholar]
- Koster F. T., Kirkpatrick T. L., Rowatt J. D., Baca O. G. Antibody-dependent cellular cytotoxicity of Coxiella burnetii-infected J774 macrophage target cells. Infect Immun. 1984 Jan;43(1):253–256. doi: 10.1128/iai.43.1.253-256.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. H., Powell J. I., Sharrow S. O., Schultz A. R. Rapid data collection, analysis, and graphics for flow microfluorometry instrumentation. Rev Sci Instrum. 1978 Aug;49(8):1137–1142. doi: 10.1063/1.1135535. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Lundin L. B., Dolan T. T., Stagg D. A. Cell-mediated immunity to Theileria-transformed cell lines. Nature. 1979 Oct 25;281(5733):678–680. doi: 10.1038/281678a0. [DOI] [PubMed] [Google Scholar]
- Plata F., Kalil J., Zilber M. T., Fellous M., Lévy D. Identification of a viral antigen recognized by H-2-restricted cytolytic T lymphocytes on a murine leukemia virus-induced tumor. J Immunol. 1983 Nov;131(5):2551–2556. [PubMed] [Google Scholar]
- Rollwagen F. M., Mathieson B. J., Asofsky R. Positive selection of T-cell subsets. I. Proliferative responses of Lyt 2 separated thymocytes and splenic T cells. Immunology. 1982 May;46(1):49–58. [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]