Abstract
A specific gene probe for the Vibrio parahaemolyticus thermostable direct hemolysin gene was constructed and used to examine the presence or absence of the thermostable direct hemolysin gene or related DNA sequences in V. parahaemolyticus and other vibrios by the DNA colony hybridization method. The gene probe consisted of a 406-base-pair, completely internal fragment covering 71% of the structural gene with PstI linkers added to the ends. Six copies of this 415-base-pair PstI fragment were cloned into plasmid pBR322, which yielded large amounts of the probe DNA. One hundred forty-one V. parahaemolyticus strains were tested with the gene probe, and the results were compared with those of phenotypic assays for the thermostable direct hemolysin. All Kanagawa phenomenon-positive strains were gene positive. However, 86% of the strains that exhibited weak Kanagawa phenomenon and 16% of Kanagawa phenomenon-negative strains also reacted with the gene probe. Immunological methods for the detection of the thermostable direct hemolysin (modified Elek test, enzyme-linked immunosorbent assay) showed better correlation with gene probe results. All gene-positive strains produced hemolysin detectable in the enzyme-linked immunosorbent assay, although occasional strains showed weak reaction. The modified Elek test was slightly less sensitive than the enzyme-linked immunosorbent assay. All gene-negative strains were also negative in these immunological assays. One hundred twenty-one strains of Vibrio spp. other than V. parahaemolyticus were tested with the gene probe; only Vibrio hollisae strains reacted with the probe under stringent conditions.
Full text
PDF
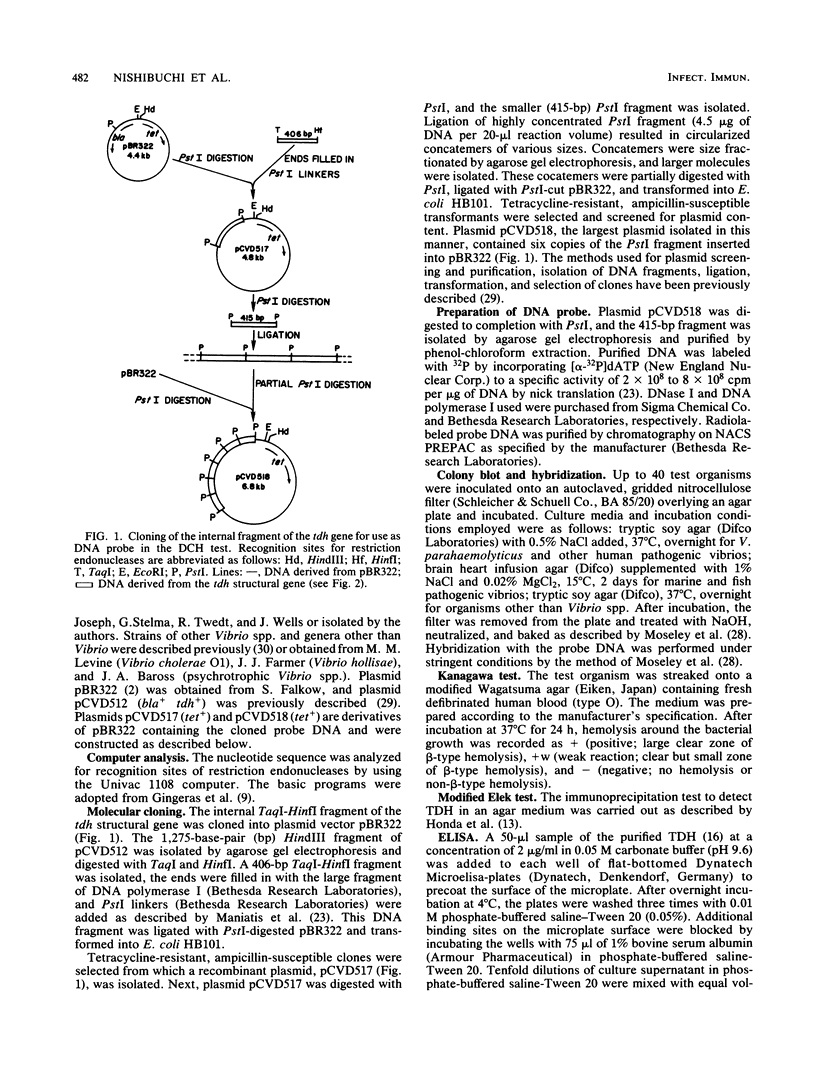
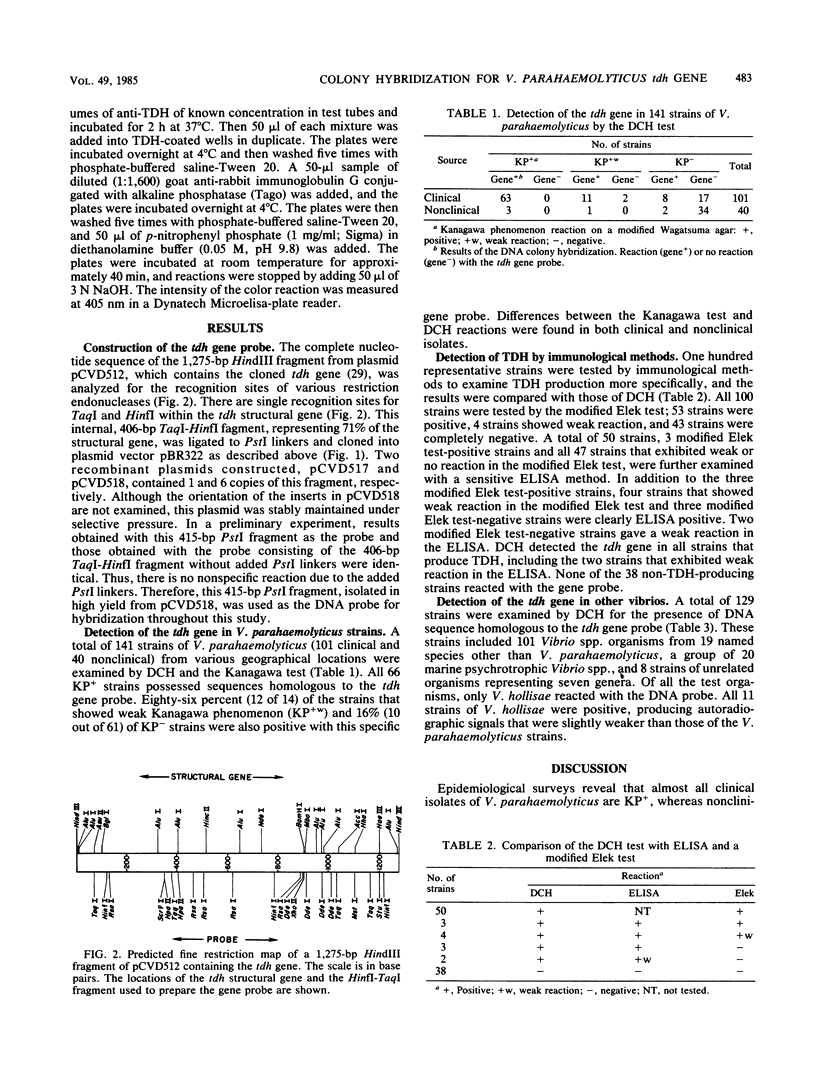



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burstyn D. G., McNicol L. A., Voll M. J. Isolation and characterization of spontaneously arising auxotrophic and Kanagawa phenomenon-negative mutants of Vibrio parahaemolyticus. Infect Immun. 1980 Mar;27(3):889–896. doi: 10.1128/iai.27.3.889-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D., Chung J. K., Tak R., Seol S. Y. Nature of the Kanagawa phenomenon of Vibrio parahaemolyticus. Infect Immun. 1975 Jul;12(1):81–87. doi: 10.1128/iai.12.1.81-87.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciufecu C., Nacescu N., Israil A. The newly described pathogenic species Vibrio mimicus isolated from human diarrhoeal stools and from a sea water sample. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Mar;254(1):89–94. [PubMed] [Google Scholar]
- Gingeras T. R., Milazzo J. P., Sciaky D., Roberts R. J. Computer programs for the assembly of DNA sequences. Nucleic Acids Res. 1979 Sep 25;7(2):529–545. doi: 10.1093/nar/7.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman F. W., Farmer J. J., 3rd, Hollis D. G., Fanning G. R., Steigerwalt A. G., Weaver R. E., Brenner D. J. Identification of Vibrio hollisae sp. nov. from patients with diarrhea. J Clin Microbiol. 1982 Mar;15(3):395–401. doi: 10.1128/jcm.15.3.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Payne W. L., Aulisio C. C. Detection and enumeration of virulent Yersinia enterocolitica in food by DNA colony hybridization. Appl Environ Microbiol. 1983 Sep;46(3):636–641. doi: 10.1128/aem.46.3.636-641.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Payne W. L., Crouch R. J., Davis V. M., English L. L., Ferreira J. L., Gemski P., Jagow J. A., Moseley S. L., Noah C. W. Genetic methods for the detection of microbial pathogens. Identification of enterotoxigenic Escherichia coli by DNA colony hybridization: collaborative study. J Assoc Off Anal Chem. 1984 Jul-Aug;67(4):801–807. [PubMed] [Google Scholar]
- Honda T., Chearskul S., Takeda Y., Miwatani T. Immunological methods for detection of Kanagawa phenomenon of Vibrio parahaemolyticus. J Clin Microbiol. 1980 Jun;11(6):600–603. doi: 10.1128/jcm.11.6.600-603.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979 Dec;26(3):1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Shimizu M., Takeda Y., Miwatani T. Isolation of a factor causing morphological changes of chinese hamster ovary cells from the culture filtrate of Vibrio parahaemolyticus. Infect Immun. 1976 Oct;14(4):1028–1033. doi: 10.1128/iai.14.4.1028-1033.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda T., Hasibuan M. A., Takeda Y., Miwatani T. Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus, and some physicochemical properties of the purified toxin. Infect Immun. 1976 Jan;13(1):133–139. doi: 10.1128/iai.13.1.133-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Calia F. M. Hemolytic reaction of clinical and environmental strains of Vibrio vulnificus. J Clin Microbiol. 1981 Oct;14(4):457–459. doi: 10.1128/jcm.14.4.457-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Campen R. K., Seidler R. J., Baldini M. M., Falkow S. Cloning of the thermostable direct or Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1984 Jul;45(1):290–292. doi: 10.1128/iai.45.1.290-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S. Cytolytic activity and virulence of Vibrio damsela. Infect Immun. 1984 May;44(2):326–331. doi: 10.1128/iai.44.2.326-331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood D. E., Kreger A. S., Richardson S. H. Detection of toxins produced by vibrio fluvialis. Infect Immun. 1982 Feb;35(2):702–708. doi: 10.1128/iai.35.2.702-708.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Miller H. G., Wilson R., Tacket C. O., Hollis D. G., Hickman F. W., Weaver R. E., Blake P. A. Illness caused by Vibrio damsela and Vibrio hollisae. Lancet. 1982 Jun 5;1(8284):1294–1297. doi: 10.1016/s0140-6736(82)92853-7. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Echeverria P., Seriwatana J., Tirapat C., Chaicumpa W., Sakuldaipeara T., Falkow S. Identification of enterotoxigenic Escherichia coli by colony hybridization using three enterotoxin gene probes. J Infect Dis. 1982 Jun;145(6):863–869. doi: 10.1093/infdis/145.6.863. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Hardy J. W., Hug M. I., Echeverria P., Falkow S. Isolation and nucleotide sequence determination of a gene encoding a heat-stable enterotoxin of Escherichia coli. Infect Immun. 1983 Mar;39(3):1167–1174. doi: 10.1128/iai.39.3.1167-1174.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Nishibuchi M., Kaper J. B. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J Bacteriol. 1985 May;162(2):558–564. doi: 10.1128/jb.162.2.558-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J. Rapid microimmunodiffusion method with species-specific antiserum raised to purified antigen for identification of Vibrio vulnificus. J Clin Microbiol. 1985 Jan;21(1):102–107. doi: 10.1128/jcm.21.1.102-107.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Chen M. E., Holmes R. K., Kaper J., Levine M. M. Environmental and human isolates of Vibrio cholerae and Vibrio parahaemolyticus produce a Shigella dysenteriae 1 (Shiga)-like cytotoxin. Lancet. 1984 Jan 14;1(8368):77–78. doi: 10.1016/s0140-6736(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Peters S., Baross J. A., Morita R. Y. Partial purification and characterization of hemolysin from a psychrotrophic kanagawa-positive marine Vibrio. Appl Environ Microbiol. 1982 Jan;43(1):39–49. doi: 10.1128/aem.43.1.39-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K., Kato T., Obara Y., Yamai S. Studies on the enteropathogenic, facultatively halophilic bacterium, Vibrio parahaemolyticus. 3. Enteropathogenicity. Jpn J Med Sci Biol. 1968 Oct;21(5):325–331. doi: 10.7883/yoken1952.21.325. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A., Carrington G. O. Halophilic vibrios from extraintestinal lesions in man. Infection. 1973;1(1):54–58. doi: 10.1007/BF01638258. [DOI] [PubMed] [Google Scholar]


