Abstract
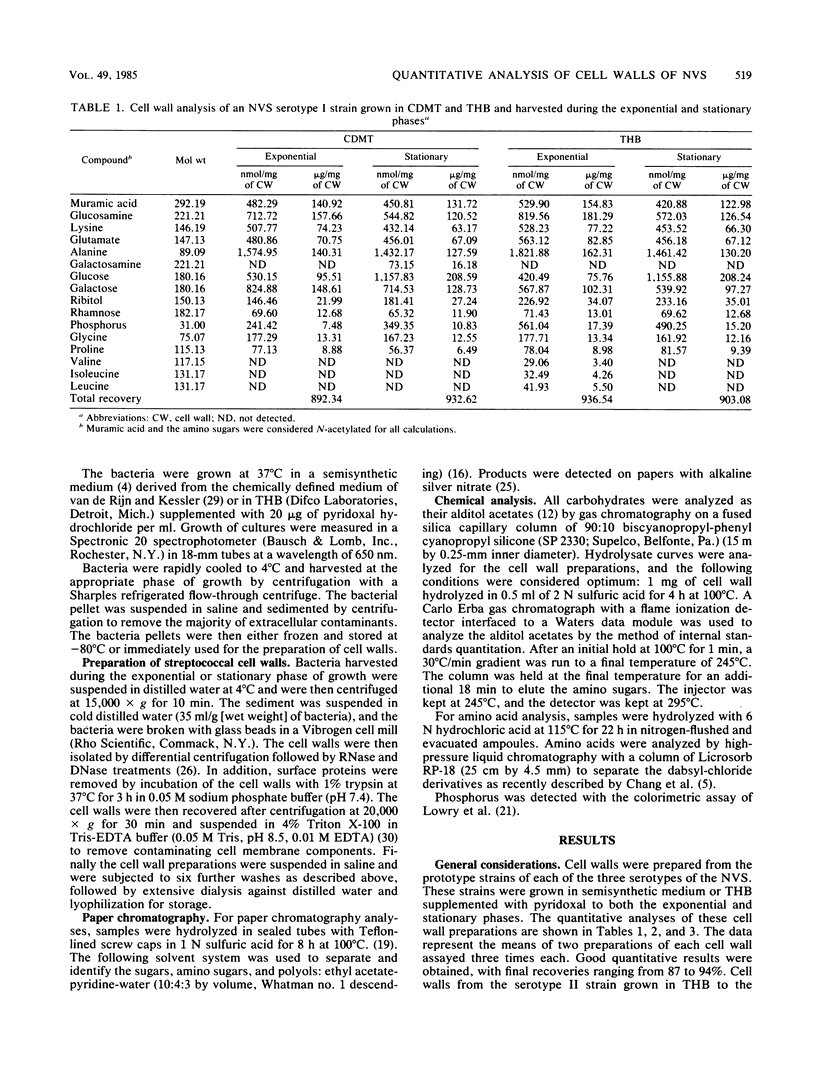
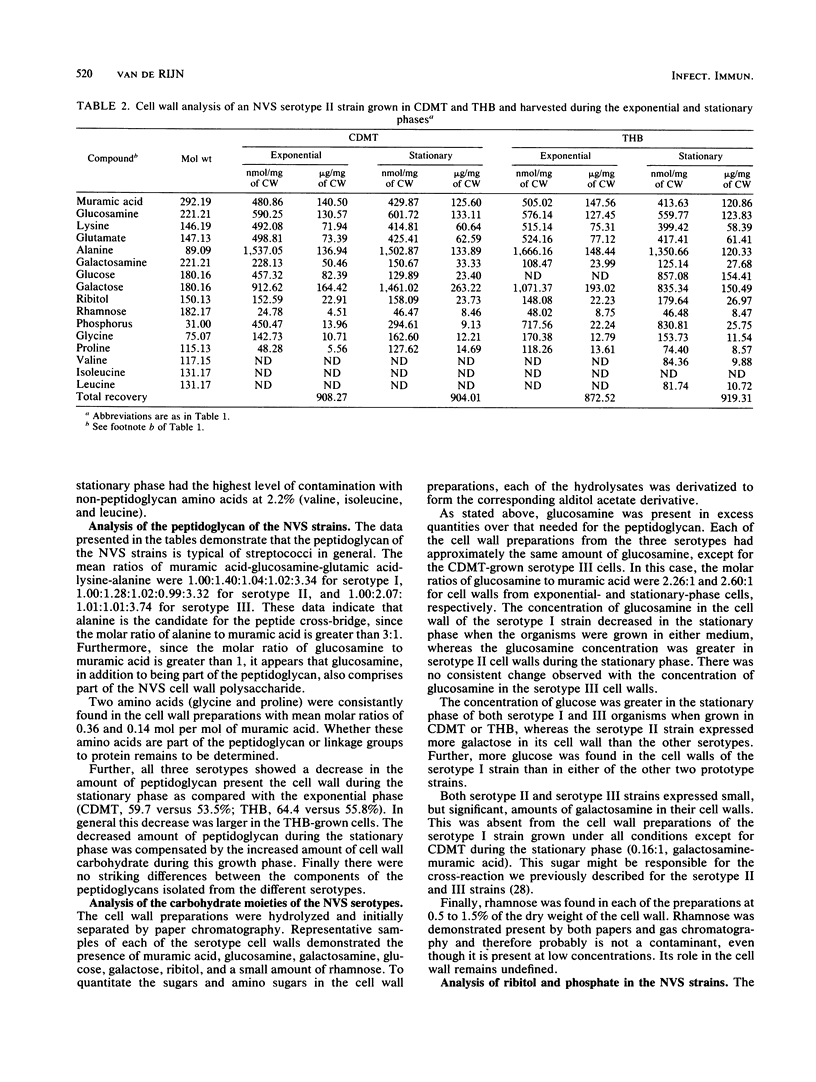
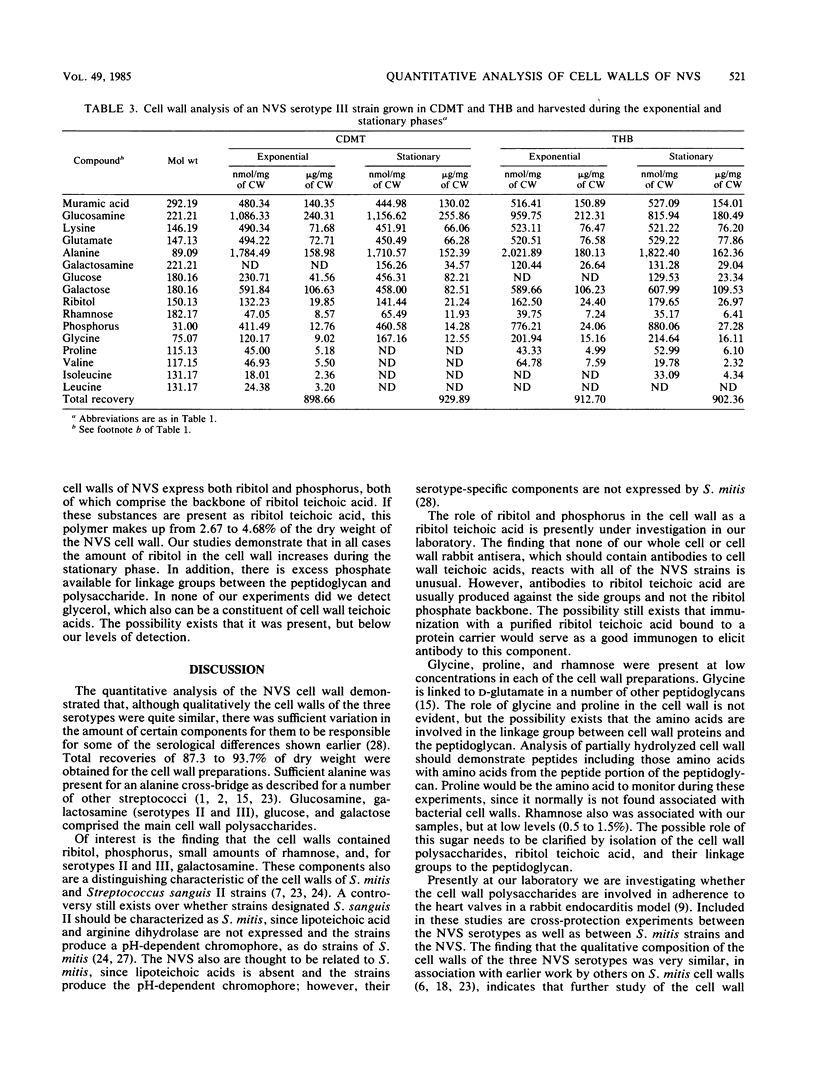
Strains of nutritionally variant streptococci are usually isolated from patients with subacute bacterial endocarditis. Only recently have these strains been subdivided into three serotypes; however, no group-specific antigen has been described. To understand the immunochemical basis for the serology of these microorganisms as well as set the groundwork for adherence studies, quantitative analysis of the cell walls of nutritionally variant streptococci was undertaken. The bacteria were grown in semisynthetic medium or pyridoxal-supplemented Todd-Hewitt broth and harvested during the exponential or stationary phase. Cell walls were isolated and analyzed for amino sugars, sugars, polyalcohols, amino acids, and phosphorus by gas chromatography, high-pressure liquid chromatography, or colorimetric assays. The peptidoglycans of the cell walls of the prototype strains from the three serotypes were representative of other streptococcal cell walls, including the presence of alanine as the possible cross-bridge. The composition of the peptidoglycan was similar for all three strains and included a decreased concentration of peptidoglycan in their cell walls during the stationary phase. Glucosamine, glucose, galactose, ribitol, and a small amount of rhamnose were found in each of the cell wall polysaccharides. Galactosamine was only found in serotype II and III cell walls and might be responsible for the previously described cross-reaction between these strains. The concentration of the other sugars and amino sugars varied in each of the cell wall preparations, depending on the growth conditions. Finally, all three strains expressed both ribitol and phosphorus in their cell walls, characteristic of the presence of a ribitol teichoic acid. Therefore the cell wall composition of the nutritionally variant streptococci varies depending on the growth conditions, and their composition appears similar to that of strains of Streptococcus mitis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiweis A. S., Craig R. A., Zinner D. D., Jablon J. M. Chemical composition of purified cell walls of cariogenic streptococci. Infect Immun. 1971 Jan;3(1):189–191. doi: 10.1128/iai.3.1.189-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet A., van de Rijn I., McCarty M. Nutritionally variant streptococci from patients with endocarditis: growth parameters in a semisynthetic medium and demonstration of a chromophore. J Bacteriol. 1981 Jun;146(3):1075–1082. doi: 10.1128/jb.146.3.1075-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R., Braun D. G. Amino acid analysis at the picomole level. Application to the C-terminal sequence analysis of polypeptides. Biochem J. 1981 Dec 1;199(3):547–555. doi: 10.1042/bj1990547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G., Williams R. E. The cell walls of streptococci. J Gen Microbiol. 1965 Dec;41(3):375–387. doi: 10.1099/00221287-41-3-375. [DOI] [PubMed] [Google Scholar]
- Cooksey R. C., Thompson F. S., Facklam R. R. Physiological characterization of nutritionally variant streptococci. J Clin Microbiol. 1979 Sep;10(3):326–330. doi: 10.1128/jcm.10.3.326-330.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- FRENKEL A., HIRSCH W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature. 1961 Aug 12;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- Fox A., Morgan S. L., Hudson J. R., Zhu Z. T., Lau P. Y. Capillary gas chromatographic analysis of alditol acetates of neutral and amino sugars in bacterial cell walls. J Chromatogr. 1983 Feb 18;256(3):429–438. doi: 10.1016/s0021-9673(01)88260-1. [DOI] [PubMed] [Google Scholar]
- George R. H. The isolation of symbiotic streptococci. J Med Microbiol. 1974 Feb;7(1):77–83. doi: 10.1099/00222615-7-1-77. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Kalonaros I. V., Bahn A. N. Antigenic composition of the cell wall of Streptococcus mitis. Arch Oral Biol. 1965 Jul-Aug;10(4):625–633. doi: 10.1016/0003-9969(65)90008-7. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Jacques N. A., Campbell L. K., Wicken A. J., Hurst S. F., Bleiweis A. S. Phenotypic stability of the cell wall of Streptococcus mutans Ingbritt grown under various conditions. Infect Immun. 1979 Dec;26(3):1071–1078. doi: 10.1128/iai.26.3.1071-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lerner P. I., Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. 1966 Jan 27;274(4):199–contd. doi: 10.1056/NEJM196601272740407. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Rosan B. Absence of glycerol teichoic acids in certain oral streptococci. Science. 1978 Sep 8;201(4359):918–920. doi: 10.1126/science.684416. [DOI] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S., Zabriskie J. B. Antigens in Streptococcus mutans cross reactive with human heart muscle. J Dent Res. 1976 Apr;55(Spec No):C59–C64. doi: 10.1177/002203457605500326011. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Identification of a Streptococcus salivarius cell wall component mediating coaggregation with Veillonella alcalescens V1. Infect Immun. 1981 May;32(2):723–730. doi: 10.1128/iai.32.2.723-730.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Ayres A., Campbell L. K., Knox K. W. Effect of growth conditions on production of rhamnose-containing cell wall and capsular polysaccharides by strains of Lactobacillus casei subsp. rhamnosus. J Bacteriol. 1983 Jan;153(1):84–92. doi: 10.1128/jb.153.1.84-92.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Bouvet A. Characterization of a pH-dependent chromophore from nutritionally variant streptococci. Infect Immun. 1984 Jan;43(1):28–31. doi: 10.1128/iai.43.1.28-31.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., George M. Immunochemical study of nutritionally variant streptococci. J Immunol. 1984 Oct;133(4):2220–2225. [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



