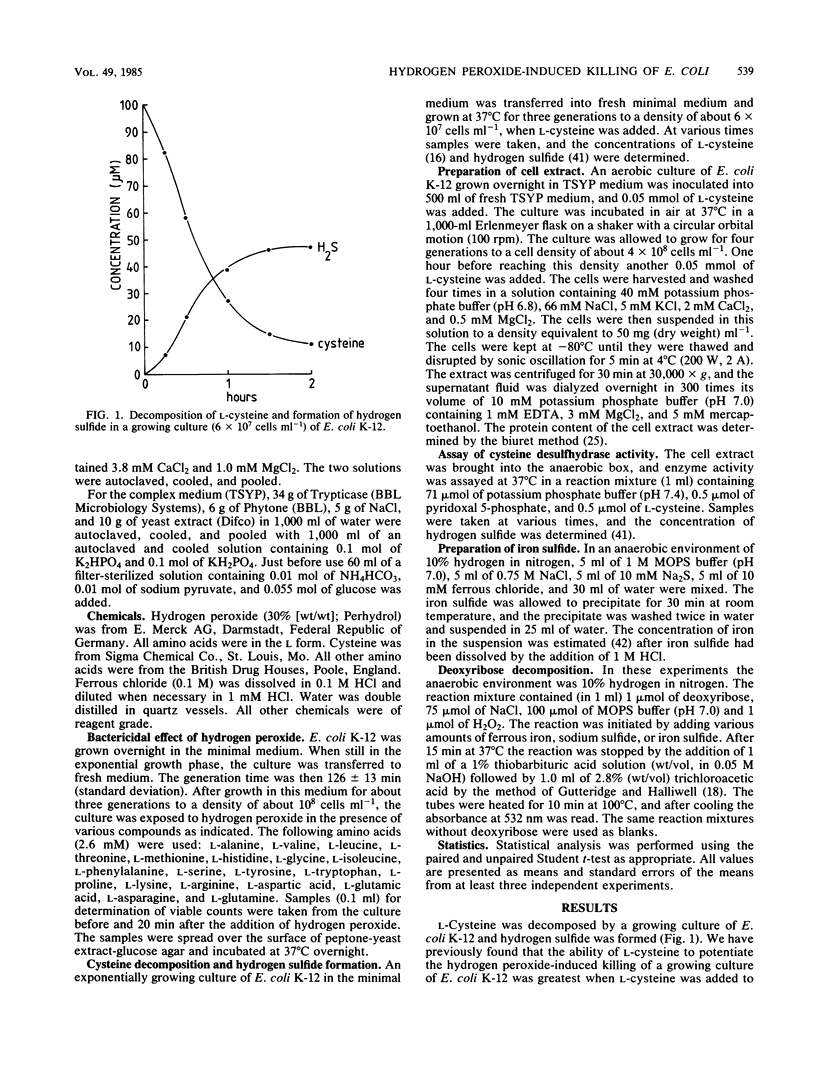
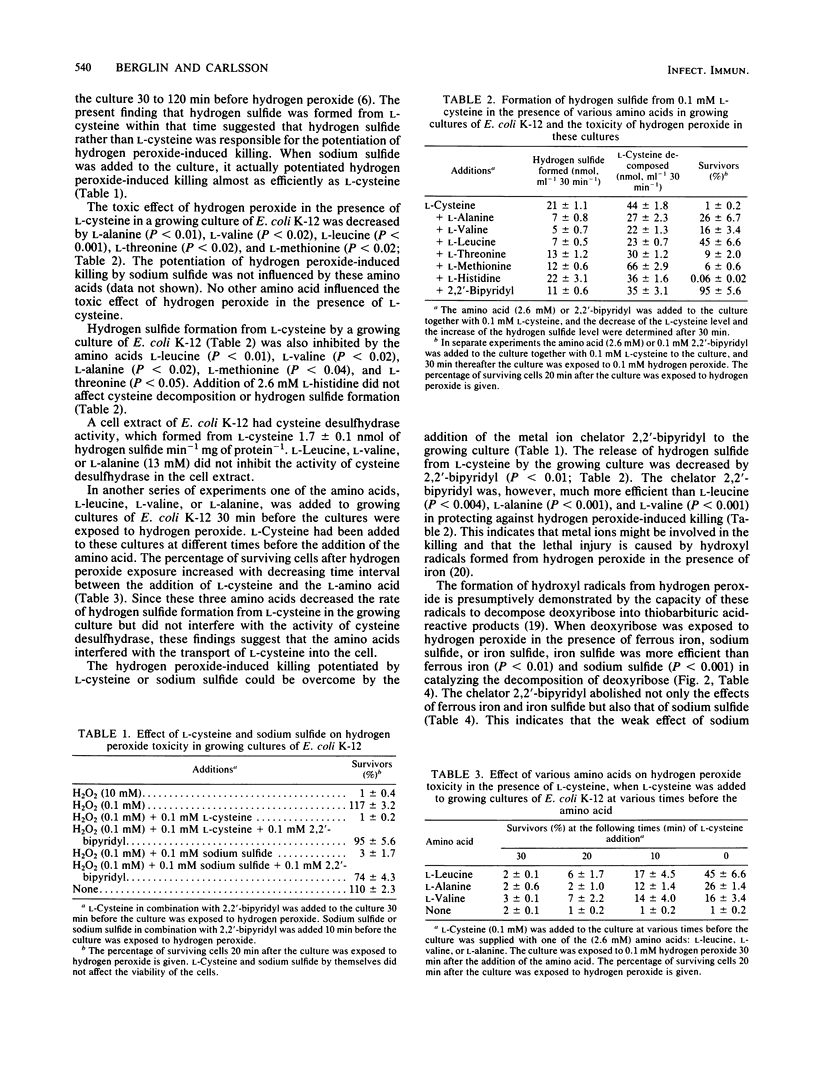
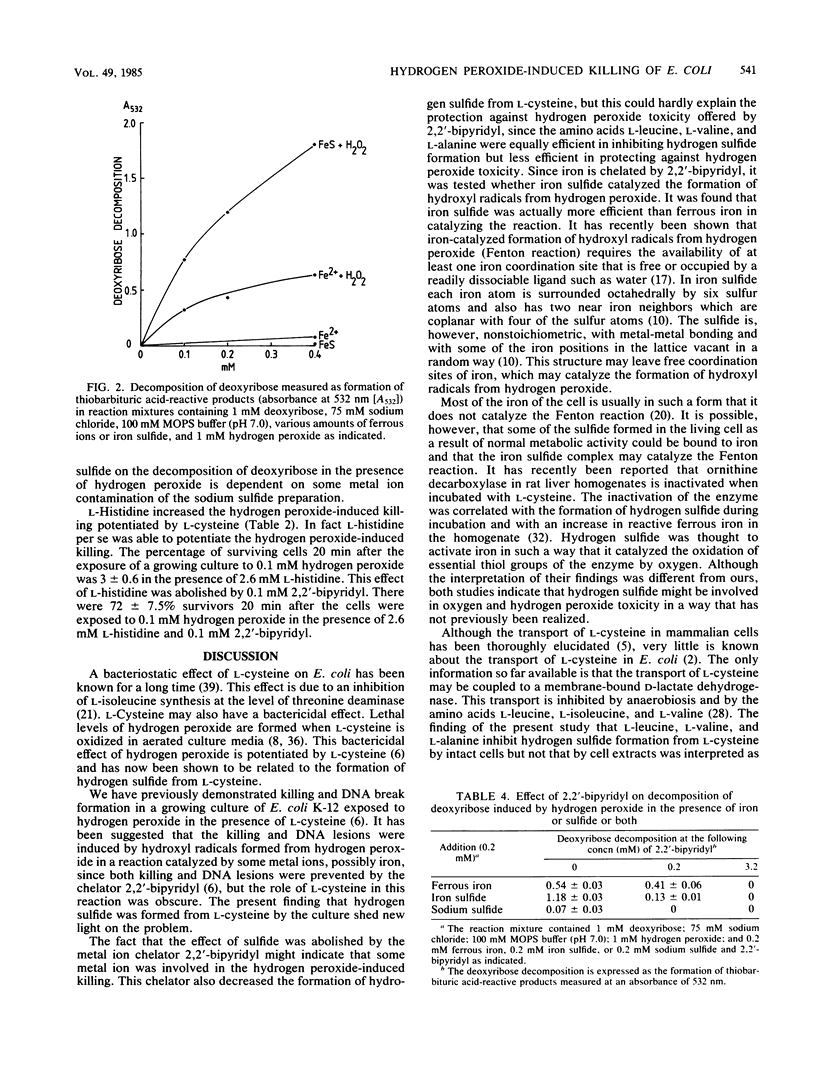
Abstract
L-Cysteine potentiates 100-fold the hydrogen peroxide-induced killing of a growing culture of Escherichia coli K-12 (Berglin et al., J. Bacteriol. 152:81-88). In the present study it is shown that hydrogen sulfide is formed from L-cysteine and that sodium sulfide could substitute for L-cysteine in the potentiation of hydrogen peroxide-induced killing of E. coli K-12. Addition of an amino acid, L-leucine, L-valine, or L-alanine, to an L-cysteine-containing medium with a growing culture of E. coli K-12 inhibited hydrogen sulfide formation and the potentiation of hydrogen peroxide-induced killing. These amino acids did not inhibit hydrogen sulfide formation from L-cysteine by a cell extract, and they did not inhibit the potentiation by sulfide of hydrogen peroxide-induced killing. This indicated that the amino acids protected the culture from L-cysteine-potentiated, hydrogen peroxide-induced killing by inhibiting the transport of L-cysteine into the cell. The potentiation by sodium sulfide of hydrogen peroxide-induced killing was abolished by the metal ion chelator 2,2'-bipyridyl. This indicated that metal ions, in addition to sulfide, were involved in the killing. Toxic effects of hydrogen peroxide are often presumed to be mediated by hydroxyl radicals formed in iron-catalyzed reactions. It was demonstrated that iron sulfide was more efficient than ferrous iron in catalyzing the formation of hydroxyl radicals from hydrogen peroxide. It was suggested that hydrogen sulfide formed in polymicrobial infections may play an important role in the host defense by potentiating the antimicrobial effect of hydrogen peroxide produced by phagocytic cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A. Quantitative studies of the avidity of naturally occurring substances for trace metals. II. Amino-acids having three ionizing groups. Biochem J. 1952 Mar;50(5):690–697. doi: 10.1042/bj0500690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984 Sep 3;779(3):289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Berglin E. H., Edlund M. B., Nyberg G. K., Carlsson J. Potentiation by L-cysteine of the bactericidal effect of hydrogen peroxide in Escherichia coli. J Bacteriol. 1982 Oct;152(1):81–88. doi: 10.1128/jb.152.1.81-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Carpenter V. S. The recA+ gene product is more important than catalase and superoxide dismutase in protecting Escherichia coli against hydrogen peroxide toxicity. J Bacteriol. 1980 Apr;142(1):319–321. doi: 10.1128/jb.142.1.319-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. A. DNA-ferrous iron catalyzed hydroxyl free radical formation from hydrogen peroxide. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1209–1215. doi: 10.1016/0006-291x(81)90748-8. [DOI] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The role of the superoxide and hydroxyl radicals in the degradation of DNA and deoxyribose induced by a copper-phenanthroline complex. Biochem Pharmacol. 1982 Sep 1;31(17):2801–2805. doi: 10.1016/0006-2952(82)90136-8. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Toeg D. Iron-dependent free radical damage to DNA and deoxyribose. Separation of TBA-reactive intermediates. Int J Biochem. 1982;14(10):891–893. doi: 10.1016/0020-711x(82)90071-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. L. Cysteine and growth inhibition of Escherichia coli: threonine deaminase as the target enzyme. J Bacteriol. 1981 Feb;145(2):1031–1035. doi: 10.1128/jb.145.2.1031-1035.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. S., Eisenstark A. Synergistic killing of Escherichia coli by near-UV radiation and hydrogen peroxide: distinction between recA-repairable and recA-nonrepairable damage. J Bacteriol. 1978 Feb;133(2):769–774. doi: 10.1128/jb.133.2.769-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. E., Mello-Filho A. C., Meneghini R. Correlation between cytotoxic effect of hydrogen peroxide and the yield of DNA strand breaks in cells of different species. Biochim Biophys Acta. 1984 Apr 5;781(3):234–238. doi: 10.1016/0167-4781(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Ismail G., Sawyer W. D., Wegener W. S. Effect of hydrogen peroxidase and superoxide radical on viability of Neisseria gonorrhoeae and related bacteria. Proc Soc Exp Biol Med. 1977 Jun;155(2):264–269. doi: 10.3181/00379727-155-39786. [DOI] [PubMed] [Google Scholar]
- Levine R. L. Mixed-function oxidation of histidine residues. Methods Enzymol. 1984;107:370–376. doi: 10.1016/0076-6879(84)07025-7. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984 Feb;157(2):622–626. doi: 10.1128/jb.157.2.622-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Hoffmann M. E. The damaging action of hydrogen peroxide on DNA of human fibroblasts is mediated by a non-dialyzable compound. Biochim Biophys Acta. 1980 Jun 27;608(1):167–173. doi: 10.1016/0005-2787(80)90144-6. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Kameji T., Hayashi S. Cysteine-dependent inactivation of hepatic ornithine decarboxylase. Biochem J. 1984 Jan 15;217(2):573–580. doi: 10.1042/bj2170573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I., Floyd R. A. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim Biophys Acta. 1984 Nov 9;790(3):238–250. doi: 10.1016/0167-4838(84)90028-1. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg G. K., Carlsson J. Metabolic inhibition of Peptostreptococcus anaerobius decreases the bactericidal effect of hydrogen peroxide. Antimicrob Agents Chemother. 1981 Dec;20(6):726–730. doi: 10.1128/aac.20.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg G. K., Granberg G. P., Carlsson J. Bovine superoxide dismutase and copper ions potentiate the bactericidal effect of autoxidizing cysteine. Appl Environ Microbiol. 1979 Jul;38(1):29–34. doi: 10.1128/aem.38.1.29-34.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. Interrelationships between amino-acids in the growth of coliform organisms. J Gen Microbiol. 1953 Aug;9(1):37–43. doi: 10.1099/00221287-9-1-37. [DOI] [PubMed] [Google Scholar]
- Rizzo A. A. The possible role of hydrogen sulfide in human periodontal disease. I. Hydrogen sulfide production in periodontal pockets. Periodontics. 1967 Sep-Oct;5(5):233–236. [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- SIEGEL L. M. A DIRECT MICRODETERMINATION FOR SULFIDE. Anal Biochem. 1965 Apr;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Schwartz C. E., Krall J., Norton L., McKay K., Kay D., Lynch R. E. Catalase and superoxide dismutase in Escherichia coli. J Biol Chem. 1983 May 25;258(10):6277–6281. [PubMed] [Google Scholar]
- Sullivan D. J. Spectrophotometric determination of ferric, ferrous, and total iron in drugs by reaction with alpha, alpha'-dipyridyl. J Assoc Off Anal Chem. 1976 Sep;59(5):1156–1161. [PubMed] [Google Scholar]


