Abstract
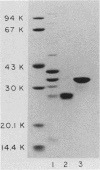
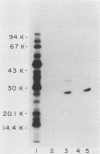
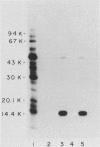
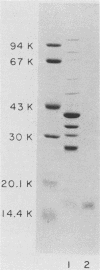
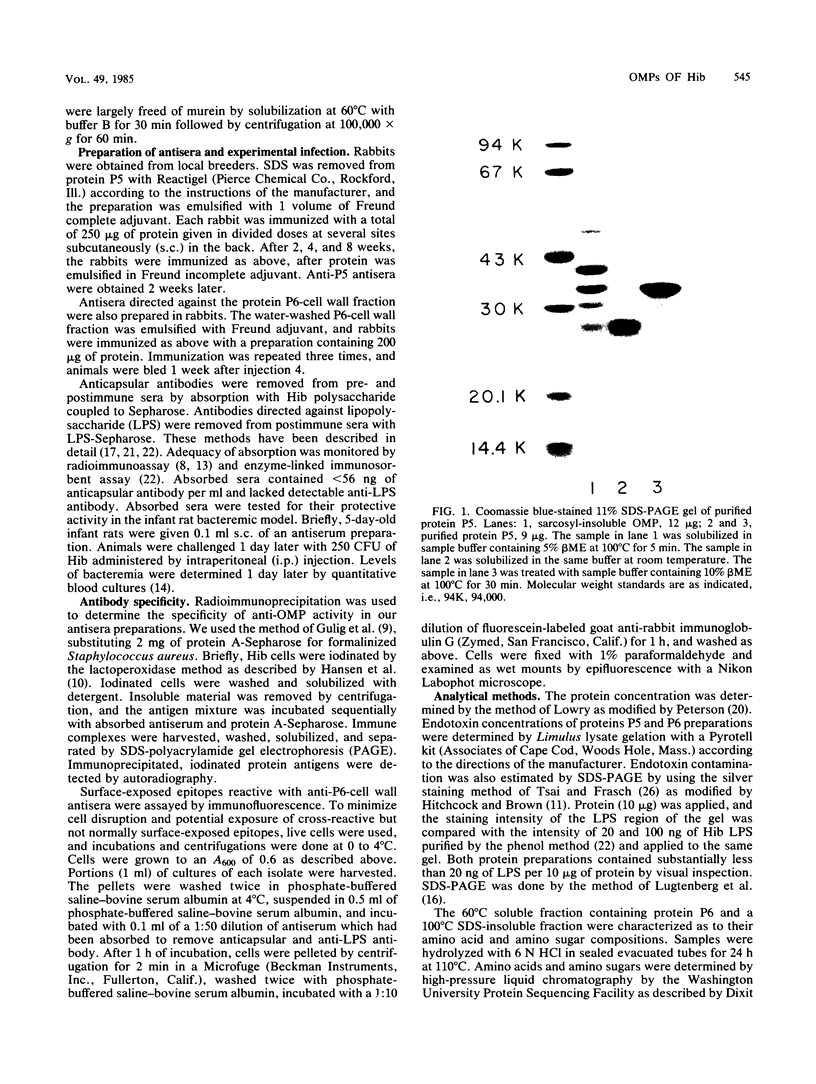
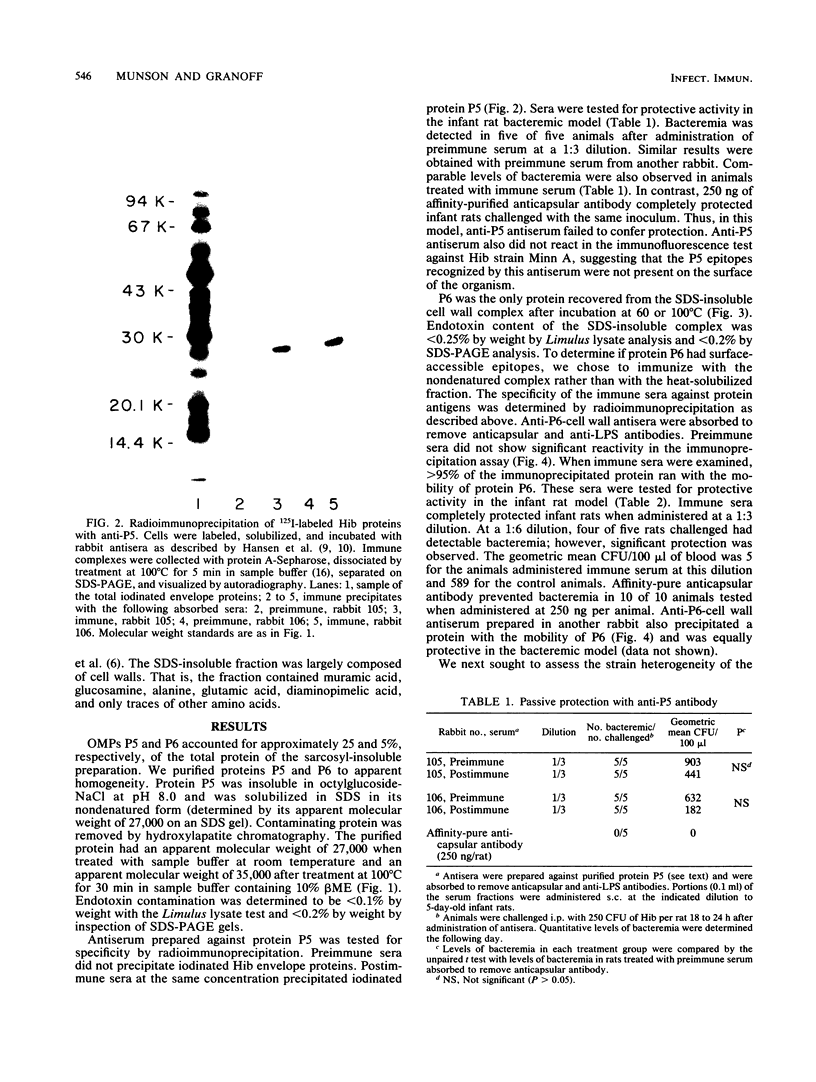
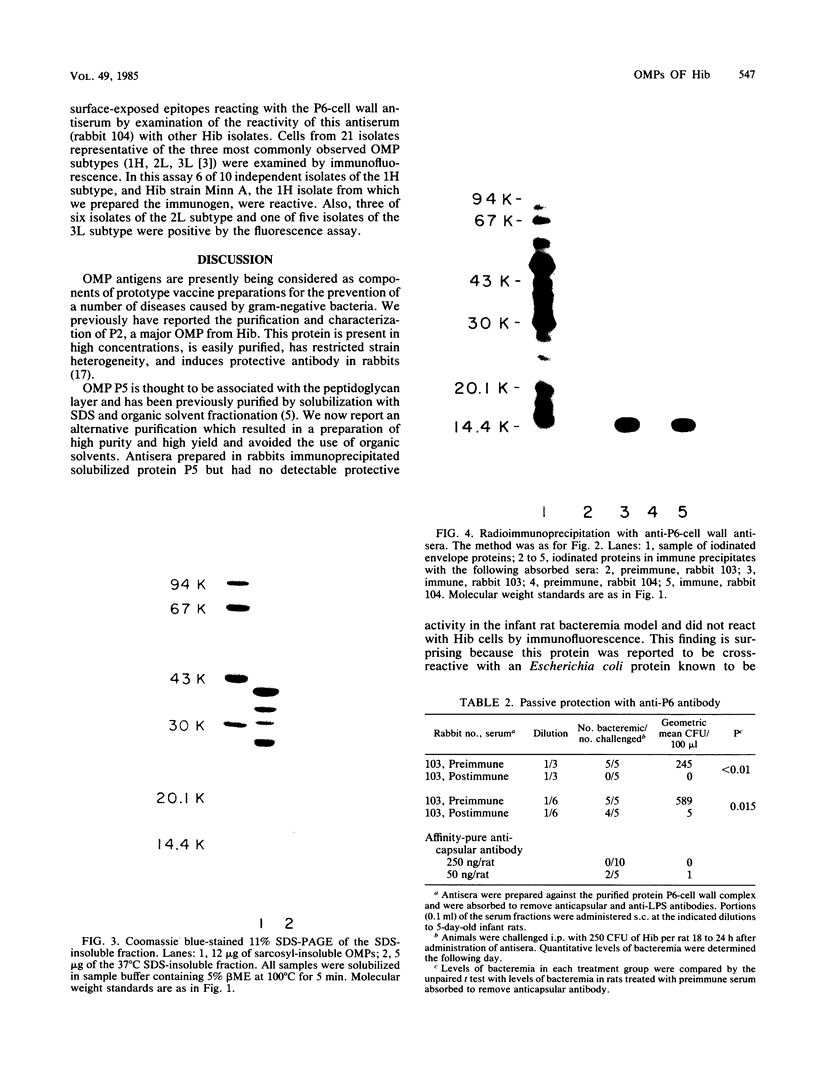
The major outer membrane proteins of Haemophilus influenzae type b (Hib), designated P5 and P6 (R.S. Munson, Jr., J.L. Shenep, S.J. Barenkamp, and D.M. Granoff, J. Clin. Invest. 72:677-684, 1983), were purified to homogeneity and partially characterized. P5 was insoluble in octylglucoside-NaCl and could be extracted with 1% sodium dodecyl sulfate (SDS) in 20 mM phosphate (pH 7.5). Solubilized P5 was further purified on hydroxylapatite in 0.1% SDS. The purified protein had an apparent molecular weight of 27,000 as determined by SDS-polyacrylamide gel electrophoresis after sample preparation at room temperature. The protein migrated with an apparent molecular weight of 35,000 after heating for 30 min at 100 degrees C in the presence of 10% beta-mercaptoethanol (beta ME). Rabbit antisera prepared against the purified preparation immunoprecipitated solubilized protein P5 but had no protective activity in the infant rat bacteremic model. The SDS-insoluble residue was further extracted with 1% SDS-0.5 M NaCl-0.1% beta ME at 37 degrees C. A single outer membrane protein, designated P6, with an apparent molecular weight of 16,000, remained insoluble under these conditions. Antiserum prepared against this insoluble fraction contained antibodies which, after removal of anti-lipopolysaccharide antibody, immunoprecipitated P6 and protected infant rats challenged with Hib. Protein P6 could be released from the insoluble cell wall in the presence of SDS-NaCl-beta ME at 60 degrees C. Thus, proteins P5 and P6 could be purified from the cell envelope of Hib. Based on the results from infant rat passive protection experiments, antigens in the P6-cell wall fraction merit further investigation as possible vaccine components. In contrast, epitopes on protein P5 did not appear to elicit protective antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates K. L., Marsh K. H., Granoff D. M. Serum opsonic activity after immunization of adults with Haemophilus influenzae type b-diphtheria toxoid conjugate vaccine. Infect Immun. 1985 Apr;48(1):183–189. doi: 10.1128/iai.48.1.183-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Wan D. T. The outer membrane of haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983 Feb;29(2):280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Boies E. G., Munson R. S., Jr Immunogenicity of Haemophilus influenzae type b polysaccharide--diphtheria toxoid conjugate vaccine in adults. J Pediatr. 1984 Jul;105(1):22–27. doi: 10.1016/s0022-3476(84)80350-9. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. S., Monji N., Schwalbe R. S., McCoy D. W. A radioactive antigen-binding assay for the measurement of antibody to Haemophilus influenzae type b capsular polysaccharide. J Immunol Methods. 1981;43(1):35–47. doi: 10.1016/0022-1759(81)90034-x. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Waldo B., Johnston R. B., Jr Separation of serum bactericidal and opsonizing activities for Haemophilus influenzae type b. Infect Immun. 1973 Sep;8(3):488–490. doi: 10.1128/iai.8.3.488-490.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]