Abstract
Previously, we demonstrated that the inwardly rectifying K+ (Kir) channel subunit Kir7.1 is highly expressed in bovine and human retinal pigment epithelium (RPE). The purpose of this study was to determine whether any of the 14 other members of the Kir gene family are expressed in native human RPE. Conventional reverse transcription-polymerase chain reaction (RT-PCR) analysis indicated that in addition to Kir7.1, 7 other Kir channel subunits (Kir1.1, Kir2.1, Kir2.2, Kir3.1, Kir3.4, Kir4.2 and Kir6.1) are expressed in the RPE, whereas in neural retina, all 14 of the Kir channel subunits examined are expressed. The identities of RT-PCR products in the RPE were confirmed by DNA sequencing. Real-time RT-PCR analysis showed, however, that transcripts of these channels are significantly less abundant than Kir7.1 in the RPE. Western blot analysis of the Kir channel subunits detected in the RPE by RT-PCR revealed the expression of Kir2.1, Kir3.1, Kir3.4, Kir4.2, Kir6.1, and possibly Kir2.2, but not Kir1.1, in both human RPE and neural retina. Our results indicate that human RPE expresses at least 5 other Kir channel subtypes in addition to Kir7.1, suggesting that multiple members of the Kir channel family may function in this epithelium.
Keywords: human retinal pigment epithelium, gene expression, potassium channels, Kir channels
1. Introduction
Potassium (K+) channels in the RPE play a key role in the transport of ions, metabolites, and fluid between the subretinal space and choroid. At the apical membrane, K+ channels contribute to subretinal K+ homeostasis and support Na+/K+ pump and Na,K,2Cl cotransporter function by providing a pathway for K+ secretion; at the basolateral membrane, they mediate K+ efflux in the last step of active K+ absorption. In addition, K+ channels affect the apical and basolateral membrane potentials, and in doing so help determine the driving force on ion and solute flux through various channels and electrogenic cotransporters. Electrophysiological studies have established that barium and cesium-sensitive inwardly rectifying K+ (Kir) channels make up a major component of the RPE apical membrane K+ conductance (Hughes and Steinberg, 1990; Hughes et al., 1995; Hughes and Takahira, 1996; Hughes and Takahira, 1998; Shimura et al., 2001; Yuan et al., 2003a). The basolateral membrane of the RPE also has a K+ conductance, but essentially nothing is known about its properties other than it is blocked by barium (Immel and Steinberg, 1986; Joseph and Miller, 1991; Maminishkis et al., 2006).
Kir channel subunits are a widely expressed group of structurally related proteins that co-assemble to form functional homotetrameric or heterotetramic channels (Isomoto et al., 1997). Each Kir channel subunit is composed of two transmembrane α helices, M1 and M2, which are separated by a conserved pore loop that forms the selectivity filter. Molecular cloning has led to the identification of 15 different Kir channel subunits, most of which form K+ channels with various degrees of inward rectification when expressed in heterologous expression systems. Kir channel subtypes display considerable diversity in terms of regulation by intracellular ATP, polyamines, pH, G proteins, and phospholipids.
Recent evidence from a variety of approaches indicates that Kir7.1 is a major component of the apical membrane K+ conductance of bovine RPE (Shimura et al., 2001; Yuan et al., 2003; Yang et al., 2003a). Others, however, have reported that in addition to Kir7.1, Kir4.1 (Kusaka et al., 1999; Kusaka et al., 2001) and Kir6.2 (Ettaiche et al., 2001) channel subunits are expressed in rat RPE. Native human RPE cells exhibit a Kir conductance with properties consistent with Kir7.1 (Hughes and Takahira, 1996) and express Kir7.1 transcript and protein (Yang et al., 2008). Given the possibility that the expression of multiple types of Kir subunits in human RPE could give rise to different Kir channels with diverse functional properties, we carried out a comprehensive survey of Kir channel subunits in native human RPE. The present study shows that native human RPE expresses transcripts for Kir1.1, Kir2.1, Kir2.2, Kir3.1, Kir3.4, Kir4.2 and Kir6.1 and that native human neural retina expresses all 14 of the Kir subunit transcripts examined. Real-time RT-PCR analysis confirmed the expression of these 7 Kir channel subunits in the RPE and showed that they are significantly less abundant than Kir7.1. Western blot analysis of the Kir channel subunits detected in the RPE by RT-PCR, revealed the expression of Kir2.1, Kir3.1, Kir3.4, Kir4.2, Kir6.1, and possibly Kir2.2, but not Kir1.1, in both human RPE and neural retina.
Some of these results have been reported in abstract form (Yang and Hughes, 2003).
2. Materials and methods
2.1. Human tissues
Human donor eyes were obtained from the Michigan Eye Bank within 24 hours of death. The protocol adhered to the provisions of the Declaration of Helsinki for the use of human tissue in research. The tissue was rejected if the donor had a history of ocular disease or if the tissue showed gross abnormalities on visual examination. The globes were hemisected by a circumferential incision around the pars plana. The neural retinas were dissected, snap frozen, and stored at −80°C until use. After the neural retinas were collected, the RPE sheets were isolated as previously described (Buraczynska et al., 2002; Yang et al., 2003a). The isolated sheets were examined under phase-contrast microscopy to determine their integrity and confirm the absence of contaminating cells from the neural retina. The isolation of RPE from neural retina was also confirmed by the failure to detect Kir4.1 (highly expressed in Muller cells) or rhodopsin (photoreceptor marker) expression in the RPE preparations after 35 PCR cycles in RT-PCR analysis. The isolated RPE sheets were snap frozen and stored at −80°C until use.
2.2. Total RNA isolation
Total RNAs were isolated from pooled human RPE sheets from 5 donors (aged 46, 47, 47, 53 & 69, for conventional RT-PCR analysis), or RPE sheets from individual donors (aged 53, 56, 57, 70, 71 & 88, for real-time RT-PCR analysis) using Trizol reagent (Life Technologies, Inc., Rockville, MD) following the manufacturer's instructions. The concentrations of total RNAs were measured by ultraviolet spectrophotometry or fluorimetry (RediPlate™ 96 RiboGreen® RNA assay, Molecular Probes, Eugene, OR). The quality of each RNA preparation was checked by denaturing formaldehyde agarose gel electrophoresis, and only those showing intact 28S and 18S ribosomal RNA bands were used for further analysis. Human retinal RNAs were isolated and measured following the procedures described above. Human heart and kidney total RNAs were purchased from Clontech (Palo Alto, CA).
2.3. PCR primers
The primers and expected sizes of RT-PCR products for human Kir genes and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; as the endogenous control or reference) are shown in Table 1. The rhodopsin-specific primers used to test RPE samples for neural retina contamination were designed based on a cDNA sequence (GenBank accession number: NM_000539; Ensembl Gene ID: ENSG00000163914) spanning a 577 bp region, with the forward primer located within exon 2 (5'-CAT CGA GCG GTA CGT GGT GGT GTG -3') and the reverse primer located within exon 5 (5'- GCC GCA GCA GAT GGT GGT GAG C -3').
Table 1.
Primers and expected sizes of RT-PCR products
| No. | Target Gene | Primer Position |
Expected | GenBank | |
|---|---|---|---|---|---|
| Forward | Reverse | Size (bp) | Accession# | ||
| 1* | Kir1.1 | 28238-28257 | 28508-28489 | 271 | U65046 |
| 2† | Kir2.1 | 1231-1255 | 1636-1610 | 406 | NM_000891 |
| 3‡ | Kir2.1 | 826-845 | 1024-1005 | 199 | NM_000891 |
| 4† | Kir2.2 | 109-129 | 929-911 | 821 | AB074970 |
| 5‡ | Kir2.2 | 1558–1577 | 1750-1731 | 193 | NM_021012 |
| 6† | Kir2.3 | 819-838 | 1215-1196 | 397 | NM_004981 |
| 7† | Kir2.4 | 1112-1130 | 1197-1179 | 86 | AF081466 |
| 8* | Kir3.1 | 595-616 | 1154-1134 | 560 | XM_010786 |
| 9† | Kir3.2 | 608-627 | 1161-1142 | 554 | XM_048829 |
| 10† | Kir3.3 | 505-523 | 1059-1040 | 555 | XM_001277 |
| 11† | Kir3.4 | 628-647 | 1353-1334 | 726 | XM_165593 |
| 12‡ | Kir3.4 | 788-809 | 887-867 | 100 | L47208 |
| 13† | Kir4.1 | 266-290 | 1322-1300 | 1,056 | U52155 |
| 14* | Kir4.2 | 693-714 | 1121-1102 | 429 | NM_002243 |
| 15† | Kir5.1 | 649-669 | 1209-1190 | 561 | BC033038 |
| 16* | Kir6.1 | 648-669 | 1195-1176 | 548 | NM_004982 |
| 17† | Kir6.2 | 538-557 | 1022-1002 | 485 | XM_006398 |
| 18* | GAPDH | 113-131 | 468-439 | 356 | AF261085 |
Note: Used for both conventional RT-PCR and real-time RT-PCR;
Used for conventional RT-PCR;
Used for real-time RT-PCR.
2.4. Conventional RT-PCR analysis
Total RNA was treated with DNase I (DNAfree; Ambion, Austin, TX) and reverse transcribed with random decamers using reverse transcriptase (RETROscript™; Ambion, Austin, TX) following procedures outlined in the manufacturer's instructions. PCR was performed with subunit-specific primer sets (Table 1). The PCR products were generated by adding DNA polymerase (Taq DNA polymerase; Gibco BRL Life Technologies, Gaithersburg, MD or SuperTaq-Plus™; Ambion, Austin, TX) and cycled 30 times for GAPDH or 35 times for Kir subunits (1 min at 94°C, 1 min at 54°C–64°C, 0.5 –1 min at 72°C), followed by a 7 min-extension at 72°C. The RT-PCR products were separated by 1.5% – 3.0% agarose gel electrophoresis. Positive controls were used to confirm the specificity of the all the primers used (data not shown).
2.5. Real-time RT-PCR analysis
The expression of Kir channel subunits in human RPE was quantified by SYBR Green I real-time RT-PCR assay on a thermal cycler (iCycler iQ; Bio-Rad Laboratories, Hercules, CA) as previously described by Yang et al. (2008). In brief, the following master mix of the components was prepared to the indicated end-concentrations: samples (diluted synthesized cDNA corresponding to 1–100 ng total RNA), forward primer (0.25 µM), reverse primer (0.25 µM), and 1 × SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). An initial denaturation step and hot-start iTaq DNA polymerase activation (95°C for 3 min) were used, followed by 40 cycles of denaturation (94°C for 30 s) and annealing/extension (63.9°C for 60 s). Fluorescence data were acquired during the annealing/extension step. The data were analyzed with iCycler™ iQ optical system software (version 3.0a; Bio-Rad Laboratories, Hercules, CA) and a fractional cycle number at which threshold fluorescence was obtained (threshold cycle, CT). Kir channel mRNA levels were quantified using the relative standard curve method, as described previously (Yang et al., 2008).
2.6. DNA sequencing and sequence analysis
RT-PCR fragments were gel purified with QIAquick gel extraction kit (Qiagen, Valencia, CA), measured by ultraviolet spectrophotometry or fluorimetry (RediPlate™ 96 PicoGreen® dsDNA assay kit, Molecular Probes, Eugene, OR) and directly confirmed by bi-directional sequencing with the same sets of primers used for PCR. DNA sequencing was performed by the DNA Sequencing Core Facility at the University of Michigan. The cDNA sequences of amplicons were compared to published sequences using Lasergene software (DNASTAR, Madison, WI).
2.7. Antibodies
Anti-Kir1.1 (sc-10694), anti-Kir2.1 (sc-18708), anti-Kir3.1 (sc-16131), anti-Kir3.4 (sc-23635), and anti-Kir6.1 (sc-11224 and 11225) goat polyclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); anti-Kir2.1 (APC-026), anti-Kir2.2 (APC-042), anti-Kir3.1 (APC-005), anti-Kir3.4 (APC-027), and anti-Kir4.2 (APC-058) rabbit polyclonal antibodies were obtained from Alomone Laboratories (Jerusalem, Israel). Anti-Kir6.1 rabbit polyclonal antibody was a gift from William A. Coetzee (CAF-1; New York University School of Medicine, New York).
2.8. Protein preparation and Western blot analysis
Protein preparation and Western blot analysis were performed using the techniques described by Zhang et al. (2005) with minor modification. Briefly, native human RPE sheets and retina were placed into buffer A (150 mM NaCl, 20 mM Tris- HCl [pH 7.4], 2 mM EDTA, 2% sodium dodecyl sulfate (SDS) containing complete protease inhibitor cocktail (Pierce Rockford, IL) and 10 mM 2-mercaptoethanol) and homogenized. Homogenized samples were centrifuged at 15,000 × g for 10 minutes at room temperature and the supernatants were collected. Proteins were isolated from RPE sheets from 7 donors (aged 15, 38, 48, 67, 68, 72, and 86 years) and retinas from 3 donors (ages 38, 48, and 68 years) without a history of ocular disease or gross abnormalities on visual examination. Human brain and kidney proteins were purchased from Zyagen Laboratories (San Diego, CA).
Proteins were mixed in Laemmli sample buffer (62.5 mM Tris [pH 6.8], 25% glycerol, 2% SDS, 0.01% bromophenol blue, and 5% 2-mercaptoethanol; Bio-Rad, Hercules, CA). RPE and retina samples were from individuals or pooled from two donors. Twenty-five µg of human proteins were applied to 4% to 20% linear gradient Tris-HCl gel (Bio-Rad). After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) at a constant current of 350 mA for 90 minutes at 4 °C in a solution containing 25 mM Tris, 193 mM glycine, and 10% methanol. The membrane was then incubated at room temperature first with Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween 20 and then with primary antibodies at working dilutions of 1:200 (anti-Kir1.1), 1:300 (anti-Kir2.1, anti-Kir2.2, anti-Kir3.1, anti-Kir3.4, anti-Kir4.2) or 1:2000 (anti-Kir6.1). Protein loading was confirmed by stripping the blot and re-probing with anti-actin or anti-beta tubulin antibodies. The specificities of anti-Kir1.1, anti-Kir2.1, anti-Kir2.2, anti-Kir3.1, anti- Kir3.4, and anti-Kir4.2 antibodies were assessed in peptide blocking studies in which the antibodies were preabsorbed with 20-fold amount of corresponding peptide antigen before immunoblotting proteins. Because RPE was in limited supply, human brain proteins were used in preabsorption studies for all these antibodies expect for Kir1.1 and Kir3.4, in which cases human kidney or human heart proteins were used, respectively. Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies and enhanced with chemiluminescent substrate (Pierce, Rockford, IL). All results presented are representative of three separate experiments.
2.9. Statistical analysis
Data are expressed as means ± standard errors and evaluated by one-way analysis of variance (ANOVA) followed by Newman-Keul's post test. A value of P < 0.05 was considered as statistically significant.
3. Results
3.1. Expression of Kir channel subunit mRNAs in native human RPE and neural retina
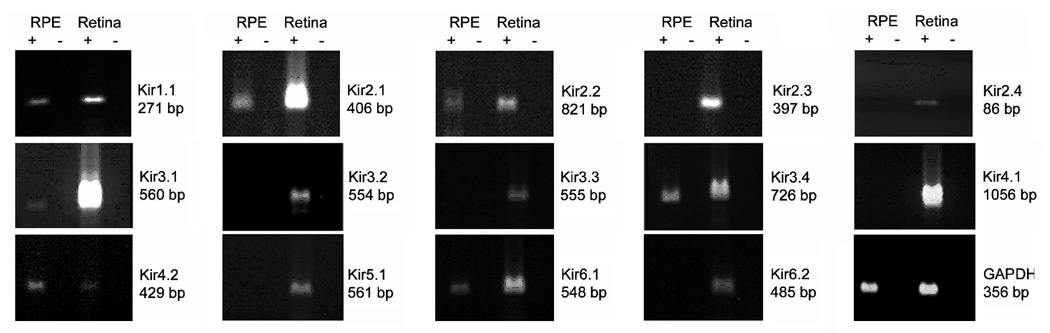
Previously, we demonstrated that the Kir7.1 is highly expressed in bovine and human RPE (Yang et al., 2003a, Yang et al., 2008). To determine whether other members of Kir channel subunits are expressed in native human RPE, we performed conventional RT-PCR analysis using 14 Kir channel subunit-specific primer sets (Table 1). The results in Figure 1 show that native human RPE expresses 7 other Kir channel subunits (Kir1.1, Kir2.1, Kir2.2, Kir3.1, Kir3.4, Kir4.2, and Kir6.1). The remaining 7 Kir channel subunits (Kir2.3, Kir2.4, Kir3.2, Kir3.3, Kir4.1, Kir5.1, and Kir6.2) were not detected in the RPE. When the same 14 primer sets were used with neural retinal RNA samples, an appropriately sized band was detected in each case (Fig. 1). Thus, although all 14 of the Kir channel subunits examined were found to be expressed in neural retina, only 7 were detected in RPE. The conventional RT-PCR analysis also indicates that the level of GAPDH transcript is less in RPE than in neural retina (Fig.1), suggesting the expression of Kir genes could be underreported in the RPE.
Figure 1. Expression of Kir channel subunits in native human RPE and neural retina.
Total RNAs isolated from pooled human RPE sheets and neural retina from five eye donors were treated with DNase I, then reverse transcribed with random decamers. RT reactions were performed in the presence (+) or absence (−) of reverse transcriptase. PCR was performed using a primer set specific for each Kir subunit or GAPDH (as endogenous control). The identities of all Kir products from the RPE were confirmed by DNA sequencing.
3.2. Quantification of Kir mRNAs in native human RPE
The Kir1.1, Kir2.1, Kir2.2, Kir3.1, Kir3.4, Kir4.2, and Kir6.1 channel subunits detected in the RPE by conventional RT-PCR were selected for further analysis by realtime RT-PCR to determine their relative abundance. Total RNA was extracted from RPE sheets isolated from individual donors and treated with DNase I to remove any residual genomic DNA. cDNA was reverse transcribed from the individual donor’s RPE RNA and used as template for real-time PCR with primers for each of the 7 Kir channel subunits and GAPDH (as an endogenous control). The specificity of RT-PCR products was confirmed by melting curve analysis, which showed single product-specific melting temperature peaks, and by agarose gel electrophoresis analysis, which yielded single product bands of the expected size (data not shown). The relative quantities of specific Kir channel subunit mRNAs in the RPE were calculated using the relative standard curve method (see Methods) in which GAPDH served as the endogenous control.
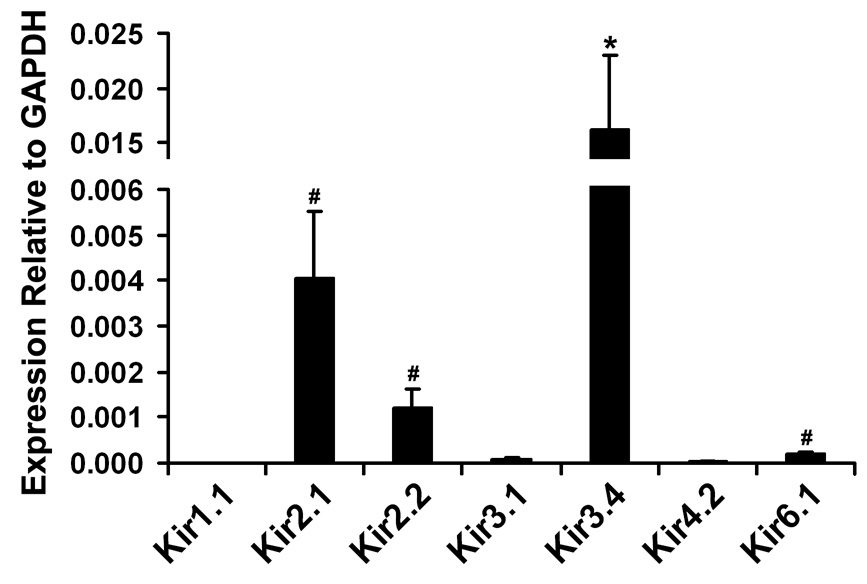
Figure 2 summarizes the relative expression of the 7 Kir channel subunits in the RPE obtained from 4–6 donors. Among these Kir channel subunits, Kir3.4 appeared to be the most abundant, but this difference was not statistically significant except in comparison to Kir1.1, Kir3.1, Kir4.2 and Kir6.1 (P < 0.05). The relative abundances of Kir1.1, Kir3.1 and Kir4.2 were significantly lower than those of Kir2.1, Kir2.2 and Kir6.1 (P < 0.05), but there was no significant difference within these two groups. Combined with relative Kir7.1 expression level obtained from the same native human RPE samples (0.961 ± 0.175; Yang et al., 2008), the relative expression sequence of Kir channel subunits is Kir7.1 > Kir3.4, Kir2.1, Kir2.2, Kir6.1 > Kir3.1, Kir4.2 and Kir1.1. The expression of Kir7.1 is significantly higher than those of the 7 other Kir channel subunits (P < 0.001).
Figure 2. Relative expression level of Kir channel subunits in native human RPE.
The relative quantity of each specific Kir mRNA in the RPE was calculated using the relative standard curve method in which GAPDH served as the endogenous control (see Methods for details). Data summarize the relative expression levels of seven Kir subunits prepared from the RPE isolated from 4 to 6 individual donors. The relative expression of Kir1.1 (7.26 × 10−6 ± 2.66 × 10−6) is too low to be displayed. Each bar in the graph shows mean ± standard error for n = 4 (Kir3.1), n = 5 (Kir1.1, Kir2.1, Kir2.2 or Kir3.4), or n = 6 (Kir4.2 or Kir6.1) individual donors. *P < 0.05, compared to Kir1.1, Kir3.1, Kir4.2 or Kir6.1; #P < 0.05, compared to Kir1.1, Kir3.1 or Kir4.2.
3.3. Expression of Kir proteins in native human RPE and neural retina
Western blot analysis was performed using polyclonal antibodies against Kir1.1, Kir2.1, Kir2.2, Kir3.1, Kir3.4, Kir4.2, and Kir6.1. As shown in Figure 3a, goat anti- Kir1.1 antibody revealed a single band of ~90 kDa in the human kidney, which likely represents dimers of the Kir1.1 channel subunit. No signal was detected in either RPE or retina.
Figure 3. Expression of Kir channel subunit proteins in native human RPE and neural retina.
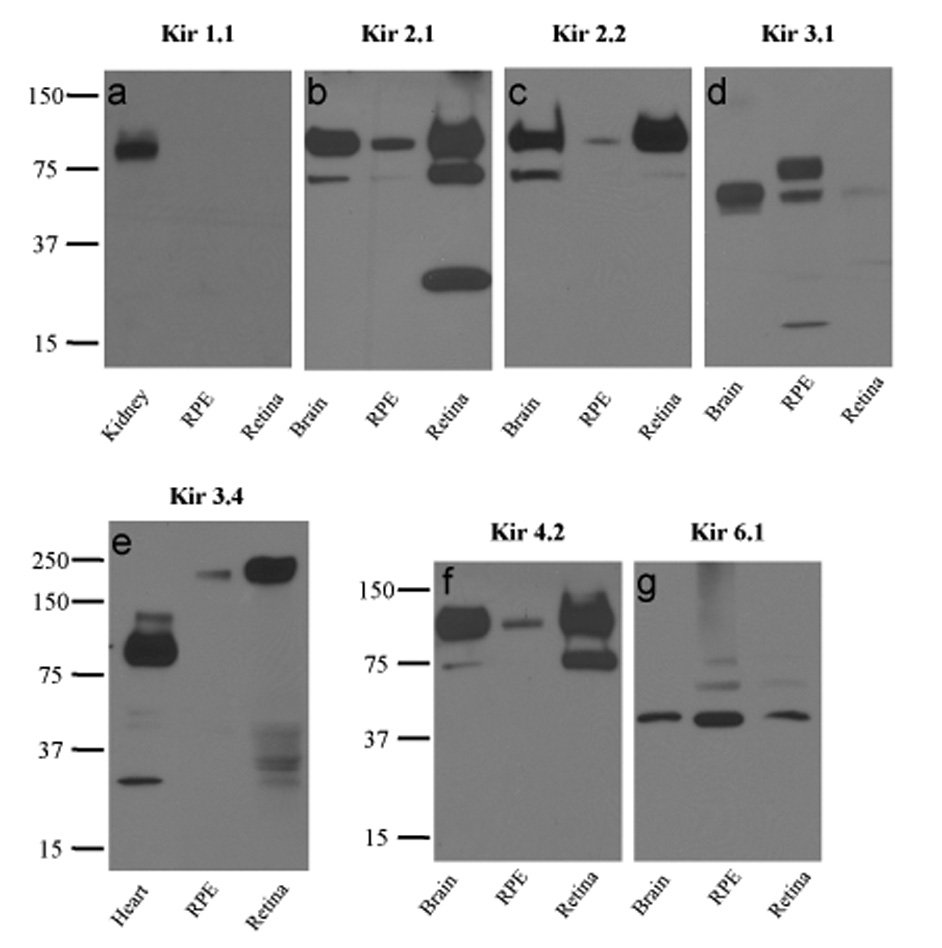
Western blots of human RPE, neural retina, and control tissue lysates probed with anti-Kir1.1 (a), anti-Kir2.1 (b), anti-Kir2.2 (c), anti-Kir3.1 (d), anti-Kir3.4 (e), anti-Kir4.2 (f), or anti-Kir6.1 (g) antibodies. In control experiments, the specificities of anti-Kir1.1, anti-Kir2.1, anti-Kir3.1, anti-Kir3.4, and anti-Kir4.2 antibodies were confirmed in peptide blocking experiments using proteins from the positive control tissues (not shown).
Rabbit anti-Kir2.1 (Fig. 3b) and anti-Kir2.2 (Fig. 3c) polyclonal antibodies, both from Alomone Labs, revealed ~100 kDa bands in brain, RPE, and retina. Because both Kir2.1 and Kir2.2 have a predicted molecular mass of 48 kDa (Giovannardi et al., 2002), the 100 kDa bands likely represent homomeric or heteromeric dimers of Kir2.1 and Kir2.2 subunits (Giovannardi et al., 2002; Zobel et al., 2003). Although the identity of the labeled protein band in brain was confirmed to be Kir2.1 in peptide blocking experiments, preabsorption of anti-Kir2.2 antibody with antigenic peptide failed to significantly reduce labeling in brain (not shown), raising questions about its specificity. The two faster-migrating bands detected in retina by the anti-Kir2.1 antibody were not characterized further. A goat anti-Kir2.1 polyclonal antibody (sc-18708; Santa Cruz Biotechnology) failed to label any band in human brain, kidney or retina (not shown).
Rabbit anti-Kir3.1 antibody (Fig. 3d) recognized a prominent ~58 kDa band in brain and RPE and a fainter ~58 kDa band in retina, consistent with detection of a Kir3.1 monomer (predicted molecular mass, 57 kDa). The slower migrating band of ~75 kDa in RPE may represent differentially glycosylated version of the core polypeptide (Torrecilla et al., 2002). A goat anti-Kir3.1 polyclonal antibody (sc-18708; Santa Cruz Biotechnology) labeled a single band <25 kDa in human kidney, brain, RPE, and retina and was judged to be non-specific (not shown).
Figure 3e shows that goat anti-Kir3.4 antibody (sc-23635, Santa Cruz Biotechnology) labeled a ~90 kDa band in heart, but in the retina and RPE, it labeled a slower migrating band of >200 kDa. Because the predicted molecular mass of human Kir3.4 is ~48 kDa, the results suggest that the band in heart represents a dimer, whereas the bands in retina and RPE represent tetramers.
When protein blots were probed with a rabbit anti-Kir4.2 antibody (Alomone Labs), bands of ~74 kDa and ~90 kDa were detected in brain and retina, but only the higher molecular mass band was detected in RPE (Fig. 3f). Kir4.2, which has been reported to have a molecular mass of ~35 kDa to 40 kDa (Ferrando-Miguel et al., 2004; Hill et al., 2002), possesses a glycosylation motif (Pearson et al., 1999; Shuck et al., 1997). Thus, the two bands in brain and retina may represent unglycosylated and glycosylated dimers (Hibino et al., 2004).
Chicken anti-Kir6.1 antibody (CAF-1) labeled ~44 kDa bands in brain, RPE and retina (Fig. 3g), consistent with the detection of Kir6.1 monomers in these tissues. This antibody has been previously characterized (Morrissey et al., 2005) and shown to label a ~44 kDa band in rat heart. We also tested two anti-Kir6.1 antibodies from Santa Cruz Biotechnology (sc-11224 and sc-11225) that were raised in goat against unique peptide sequences in the C-terminus of human Kir6.1. Neither antibody labeled protein from human kidney, brain, retina, and RPE (not shown).
In summary, Western blot analysis indicates the expression of Kir2.1, Kir3.1, Kir3.4, Kir4.2, Kir6.1, and possibly Kir2.2 subunits, but not Kir1.1 in both the RPE and neural retina.
4. Discussion
This is the first comprehensive survey of Kir channel subunits in native human RPE and neural retina. The expression of Kir channel subunits in the RPE and neural retina is considered separately below.
4.1. Kir channel subunit expression in the RPE
In a previous study (Yang et al., 2008), we demonstrated that Kir7.1 is highly expressed in human RPE, its transcript being nearly abundant as GAPDH. The high level of Kir7.1 expression is consistent with a previous estimate, based on electrophysiological measurements, that bovine RPE contains a high density of Kir7.1 channels, more than 60,000 channels or 240,000 channel subunits per cell (Shimura et al., 2001). The results of the current study show that transcripts for 7 other members of the Kir channel gene family, namely Kir1.1, Kir2.1, Kir2.2, Kir3.1, Kir3.4, Kir4.2, and Kir6.1, are also expressed, albeit at levels less than 50-fold lower than that of Kir7.1. Two Kir channel subtypes previously reported to be present in rat RPE, Kir4.1 (Kusaka et al., 1999; Kusaka et al., 2001) and Kir6.2 (Ettaiche et al., 2001), are either absent from human RPE or present at levels that are below the detection limit. The present results are consistent with the detection of transcripts for Kir2.1 and Kir6.1, but not for Kir2.4, Kir4.1 or Kir6.2, in RPE (Hughes et al., 2000; Yang et al., 2002, 2003a).
Although relatively low transcript abundance compared to Kir7.1 might seem to suggest a minor role for these Kir channel subtypes in RPE physiology, it is important to emphasize that the present data come from pooled RPE cells from different regions of the retina. Specific Kir channel subtypes might be expressed in a subset of RPE cells dispersed throughout the retina or lying within a restricted region (such as the macula), but the overall signal in our measurements would be low. Future studies are required to determine if these channel subtypes exhibit regional localization.
Consistent with RT-PCR results, we demonstrated the expression of Kir2.1, Kir3.1, Kir3.4, Kir4.2, Kir6.1, and possibly Kir2.2 channel subunits, in native human RPE and neural retina using Western blot analysis and available antibodies. However, we did not detect the expression of Kir1.1, suggesting that if Kir1.1 protein is expressed in RPE, it is at a level below the detection limit. This is consistent with the relatively low abundance of Kir1.1 mRNA transcript in the RPE (Fig. 2).
4.2. Kir channel subunit expression in neural retina
Although the focus of this study was to determine which members of the Kir channel family are expressed in human RPE, we also carried out RT-PCR and Western blot analyses of human neural retina. We recently reported the detection of Kir7.1 and its splice variant Kir7.1S in human neural retina by RT-PCR (Yang et al., 2008); the RT-PCR analysis in the present study detected all 14 remaining members of the Kir channel family in human neural retina. Western blot analysis, which was limited to those Kir channel subunits that were found by RT-PCR to be expressed in RPE, revealed the expression of Kir2.1, Kir3.1, Kir3.4, Kir4.2, Kir6.1, and possibly Kir2.2, but not Kir1.1 in neural retina. Previous immunohistochemical studies provided evidence for the expression of multiple different Kir channel subunits in rodent retina (Chen et al., 2004; Kusaka et al., 1999; Tian et al., 2003; Kofuji et al., 2002), including Kir1.1, Kir2.2, Kir2.3, Kir3.1, Kir3.2, Kir3.3, Kir4.1, Kir6.1 and Kir6.2. Previously, we cloned Kir2.4 from a human retina cDNA library and demonstrated that it is expressed in human and bovine neural retina, but not in the RPE (Hughes et al., 2000). Although it is well accepted that Kir2.1 and Kir4.1 channel subunits are the basis for the inwardly rectifying K+ channels in Müller cells (Kofuji et al., 2002), the function of other Kir channel subtypes localized to retinal neurons is controversial (Lee and Ishida, 2007).
4.3. Physiological significance
It is well established that Kir7.1 makes a major contribution to the RPE apical membrane K+ conductance, where it plays a key role in subretinal K+ homeostasis. The significance of the other Kir subtypes detected in human RPE is unclear. The RPE is a highly polarized cell and its asymmetric distribution of ion channels and transporters enable it to carry out vectorial transport. Consequently, ascertaining the subcellular localization of the other identified Kir channel subunits is key to understanding their physiological function in the RPE. In a blocker sensitivity study on intact toad RPE-choroid, we determined that millimolar concentrations of barium are necessary to block the RPE apical membrane K+ conductance (Hughes et al., 1995), consistent with the properties of Kir7.1 (Shimura et al., 2001). Because other Kir channel subtypes are about an order of magnitude more sensitive to block by barium, it seems unlikely that they contribute much to the apical K+ conductance. An intriguing possibility is that one or more of the Kir channels identified in this study are localized at the basolateral membrane, where they could mediate K+ efflux in the final step in the process of K+ reabsorption.
The Kir channel subtypes expressed in human RPE display considerable differences in their biophysical properties and regulation. Kir2.1 and Kir2.2 channels, which can exist as homomeric or heteromeric complexes (Preisig-Muller et al., 2002), are constitutively active and strongly inwardly rectifying (Kubo et al., 1993; Koyama et al., 1994; Rae and Shepard, 2000; Yang et al., 2000; Yang et al., 2003b; Zobel et al., 2003). The strong rectification of these channels allows them to stabilize the membrane potential near the potassium equilibrium potential in excitable cells and glia. At the resting membrane potentials of human RPE, (~−56 mV; Quinn and Miller, 1992), however, Kir2.1, Kir2.2, and Kir2.1/Kir2.2 channels would largely be closed due to voltage-dependent block by intracellular polyamines (Nichols and Lopatin, 1997). Interestingly, strongly inwardly rectifying K+ currents consistent with Kir2.1 or Kir2.2 have been observed in cultured human RPE cells (Hughes and Takahira, 1996; Wen et al., 1993). The significance of these findings is unclear, but one possibility is that de-differentiation of the RPE in culture leads to increased expression of Kir2.1 and/or Kir2.2 subunits and decreased expression of Kir7.1.
Kir3.1 and Kir3.4 subunits co-assemble to form G protein-activated, strongly inwardly rectifying K+ (GIRK) channels in the heart and other tissues (Wischmeyer et al., 1997). Kir3.4 channels can also exist as homotetramers, but Kir3.1 subunits cannot form functional channels alone. Kir3.1/Kir3.4 and Kir3.4 channels are directly activated by Gβγ subunits, intracellular Na+, and PIP2, and function in neurons and cardiac muscle by contributing to the resting potential and regulating excitability. In the RPE, Kir3.1/Kir3.4 channels might be activated by Gβγ subunits released following stimulation Gi/o protein-coupled receptors or by the elevation of intracellular Na+ concentration. On the other hand, these channels would be expected to be inhibited by activation of spatially proximal Gq protein-coupled receptors as a result of PIP2 depletion (Cho et al., 2005).
Kir4.2 subunits form K+ channels with intermediate inward rectification and can co-assemble with Kir5.1 subunits, which influence its rectification and gating properties (Pearson et al., 1999). Unlike Kir4.1 channels, Kir4.2 channels expressed in mammalian cells are insensitive to intracellular pH within the physiological range (Lam et al., 2006). Our results suggest that Kir4.2 channels exist as homomeric channels in the RPE, where they likely contribute to K+ efflux under resting conditions.
Kir6 subunits cannot form homomeric channels alone but co-assemble with sulfonylurea receptor (SUR) subunits to form the functional channels (Seino, 1999). The Kir6 tetrameric complex forms the channel’s pore structure whereas the SUR (SUR1, SUR2A, or SUR2B) tetramer confers its nucleotide sensitivity and aspects of its pharmacology. Kir6.1 and SUR2B subunits form vascular KATP channels (Li et al., 2003), which are activated by ATP and nucleotide diphosphates (Yamada et al., 1997). There is immunological evidence that Kir6.1 subunits may also contribute to mitochrondrial KATP channels (Cuong et al., 2005; Singh et al., 2003), but this interpretation has been challenged (Foster et al., 2008). It will be interesting to learn which SUR isoform(s) complexes with Kir6.1 in the RPE.
We recently showed that Kir7.1 channels are sensitive to intracellular pH (Hughes and Swaminathan, 2008), which is the likely basis for the increase Kir conductance in bovine RPE that occurs in response to mild intracellular acidification (Yuan et al., 2003). Given that Kir channels can exist as homomeric or heteromeric complexes, it is reasonable to ask whether Kir7.1 might co-assemble with other Kir subunits to form heteromeric channels with unique properties. Although this is a possibility, it seems unlikely because Kir7.1 is most structurally divergent member of the Kir channel family (Doring et al., 1998).
Although all Kir channel subtypes expressed in the RPE are gated by PIP2, they differ in their sensitivity: Kir2.1 has a high affinity for PIP2, whereas members of the Kir3, Kir6 and Kir7 subfamilies have relatively low affinities and, hence, can be inhibited by phospholipase C-mediated decreases in the membrane content of PIP2 (Huang et al., 1998; Zhang et al., 1999; Rohacs et al., 1999; Hilgemann and Ball, 1996; Shyng and Nichols, 1998; Baukrowitz et al., 1998; Du et al., 2004). The Kir current in bovine RPE is inhibited by ATP depletion (Hughes and Takahira, 1998), and recent evidence suggests that this is a consequence of a decrease in the regeneration of membrane PIP2 by phosphoinositol 4-kinase, a process that is dependent on ATP hydrolysis (Pattnaik and Hughes, 2006). Although Kir7.1 channels likely underlie a major component of this ATP-dependent current, the present results raise the possibility that Kir3.1/Kir3.4 or Kir6.1/SUR channels also contribute.
In summary, the present study provides the first systematic analysis of Kir channel subunits in native human RPE and demonstrates that, in addition to Kir7.1, at least 5 other Kir channel subunits are also expressed. It is tempting to speculate that the various Kir channel subtypes identified here could impact RPE function differentially depending on the metabolic or physiological state of the cell.
Acknowledgments
This work was supported by NIH Grant EY08850, Core Grant EY07703, the Foundation Fighting Blindness, and RPB Lew R. Wasserman Merit Award to BAH. The authors thank the Michigan Eye Bank (Ann Arbor, MI) for assistance in obtaining human eye tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Buraczynska M, Mears AJ, Zareparsi S, Farjo R, Filippova E, Yuan Y, MacNee SP, Hughes B, Swaroop A. Gene expression profile of native human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2002;43:603–607. [PubMed] [Google Scholar]
- Chen L, Yu YC, Zhao JW, Yang XL. Inwardly rectifying potassium channels in rat retinal ganglion cells. Eur. J. Neurosci. 2004;20:956–964. doi: 10.1111/j.1460-9568.2004.03553.x. [DOI] [PubMed] [Google Scholar]
- Cho H, Lee D, Lee SH, Ho WK. Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc. Natl. Acad. Sci. U S A. 2005;102:4643–4648. doi: 10.1073/pnas.0408844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuong DV, Kim N, Joo H, Youm JB, Chung JY, Lee Y, Park WS, Kim E, Park YS, Han J. Subunit composition of ATP-sensitive potassium channels in mitochondria of rat hearts. Mitochondrion. 2005;5:121–133. doi: 10.1016/j.mito.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Doring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J. Neurosci. 1998;18:8625–8636. doi: 10.1523/JNEUROSCI.18-21-08625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J. Biol. Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- Ettaiche M, Heurteaux C, Blondeau N, Borsotto M, Tinel N, Lazdunski M. ATP-sensitive potassium channels (KATP) in retina: a key role for delayed ischemic tolerance. Brain Res. 2001;890:118–129. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- Ferrando-Miguel R, Cheon MS, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down Syndrome brain (Part V): overexpression of phosphatidyl-inositol-glycan class P protein (DSCR5) Amino Acids. 2004;26:255–261. doi: 10.1007/s00726-004-0065-9. [DOI] [PubMed] [Google Scholar]
- Foster DB, Rucker JJ, Marban E. Is Kir6.1 a subunit of mitoKATP? Biochem. Biophys. Res. Commun. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannardi S, Forlani G, Balestrini M, Bossi E, Tonini R, Sturani E, Peres A, Zippel R. Modulation of the inward rectifier potassium channel IRK1 by the Ras signaling pathway. J. Biol. Chem. 2002;277:12158–12163. doi: 10.1074/jbc.M110466200. [DOI] [PubMed] [Google Scholar]
- Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J. Biol. Chem. 2004;279:44065–44073. doi: 10.1074/jbc.M405985200. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Hill CE, Briggs MM, Liu J, Magtanong L. Cloning, expression, and localization of a rat hepatocyte inwardly rectifying potassium channel. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G233–G240. doi: 10.1152/ajpgi.00256.2001. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Kumar G, Yuan Y, Swaminathan A, Yan D, Sharma A, Plumley L, Yang-Feng TL, Swaroop A. A. Cloning and functional expression of human retinal Kir2.4, a pH-sensitive inwardly rectifying K(+) channel. Am. J. Physiol. Cell Physiol. 2000;279:C771–C784. doi: 10.1152/ajpcell.2000.279.3.C771. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Shaikh A, Ahmad A. Effects of Ba2+ and Cs+ on apical membrane K+ conductance in toad retinal pigment epithelium. Am. J. Physiol. 1995;268:C1164–C1172. doi: 10.1152/ajpcell.1995.268.5.C1164. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Steinberg RH. Voltage-dependent currents in isolated cells of the frog retinal pigment epithelium. J. Physiol. 1990;428:273–297. doi: 10.1113/jphysiol.1990.sp018212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Takahira M. ATP-dependent regulation of inwardly rectifying K+ current in bovine retinal pigment epithelial cells. Am. J. Physiol. Cell Physiol. 1998;275:C1372–C1383. doi: 10.1152/ajpcell.1998.275.5.C1372. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Takahira M. Inwardly rectifying K+ currents in isolated human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1996;37:1125–1139. [PubMed] [Google Scholar]
- Hughes BA, Swaminathan A. Modulation of the Kir7.1 Potassium Channel by Extracellular and Intracellular pH. Am. J. Physiol. Cell Physiol. 2008;294:C423–C431. doi: 10.1152/ajpcell.00393.2007. [DOI] [PubMed] [Google Scholar]
- Immel J, Steinberg RH. Spatial buffering of K+ by the retinal pigment epithelium in frog. J. Neurosci. 1986;6:3197–3204. doi: 10.1523/JNEUROSCI.06-11-03197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn. J. Physiol. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Joseph DP, Miller SS. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J. Physiol. 1991;435:439–463. doi: 10.1113/jphysiol.1991.sp018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, Thomzig A, Veh RW, Bringmann A, Reichenbach A. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia. 2002;39:292–303. doi: 10.1002/glia.10112. [DOI] [PubMed] [Google Scholar]
- Koyama H, Morishige K, Takahashi N, Zanelli JS, Fass DN, Kurachi Y. Molecular cloning, functional expression and localization of a novel inward rectifier potassium channel in the rat brain. FEBS Lett. 1994;341:303–307. doi: 10.1016/0014-5793(94)80478-8. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Horio Y, Fujita A, Matsushita K, Inanobe A, Gotow T, Uchiyama Y, Tano Y, Kurachi Y. Expression and polarized distribution of an inwardly rectifying K+ channel, Kir4.1, in rat retinal pigment epithelium. J. Physiol. 1999;520(Pt 2):373–381. doi: 10.1111/j.1469-7793.1999.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka S, Inanobe A, Fujita A, Makino Y, Tanemoto M, Matsushita K, Tano Y, Kurachi Y. Functional Kir7.1 channels localized at the root of apical processes in rat retinal pigment epithelium. J. Physiol. 2001;531:27–36. doi: 10.1111/j.1469-7793.2001.0027j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HD, Lemay AM, Briggs MM, Yung M, Hill CE. Modulation of Kir4.2 rectification properties and pHi-sensitive run-down by association with Kir5.1. Biochim. Biophys. Acta. 2006;1758:1837–1845. doi: 10.1016/j.bbamem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lee SC, Ishida AT. Ih without Kir in adult rat retinal ganglion cells. J. Neurophysiol. 2007;97:3790–3799. doi: 10.1152/jn.01241.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J. Membr. Biol. 2003;196:61–69. doi: 10.1007/s00232-003-0625-z. [DOI] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest. Ophthalmol. Vis. Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Pearson WL, Dourado M, Schreiber M, Salkoff L, Nichols CG. Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver. J. Physiol. 1999;514:639–653. doi: 10.1111/j.1469-7793.1999.639ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik BR, Hughes BA. Regulation of inwardly rectifying K+ (Kir) channels in the retinal pigment epithelium (RPE) by phosphatidylinositol 4, 5–bisphosphate (PIP2) Invest. Ophthalmol. Vis. Sci. 2006;47 E-Abstract 4722. [Google Scholar]
- Preisig-Muller R, Schlichthorl G, Goerge T, Heinen S, Bruggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc. Natl. Acad. Sci. U S A. 2002;99:7774–7779. doi: 10.1073/pnas.102609499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1992;33:3513–3527. [PubMed] [Google Scholar]
- Rae JL, Shepard AR. Kir2.1 Potassium channels and corneal epithelia. Curr. Eye Res. 2000;20:144–152. [PubMed] [Google Scholar]
- Rohacs T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J. Biol. Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu. Rev. Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Shimura M, Yuan Y, Chang JT, Zhang S, Campochiaro PA, Zack DJ, Hughes BA. Expression and permeation properties of the K+ channel Kir7.1 in the retinal pigment epithelium. J. Physiol. 2001;531:329–346. doi: 10.1111/j.1469-7793.2001.0329i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuck ME, Piser TM, Bock JH, Slightom JL, Lee KS, Bienkowski MJ. Cloning and characterization of two K+ inward rectifier (Kir) 1.1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3) J. Biol. Chem. 1997;272:586–593. doi: 10.1074/jbc.272.1.586. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J. Mol. Cell Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Tian M, Chen L, Xie JX, Yang XL, Zhao JW. Expression patterns of inwardly rectifying potassium channel subunits in rat retina. Neurosci. Lett. 2003;345:9–12. doi: 10.1016/s0304-3940(03)00363-x. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J. Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R, Lui GM, Steinberg RH. Whole-cell K+ currents in fresh and cultured cells of the human and monkey retinal pigment epithelium. J. Physiol. 1993;465:121–147. doi: 10.1113/jphysiol.1993.sp019669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeyer E, Doring F, Wischmeyer E, Spauschus A, Thomzig A, Veh R, Karschin A. Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels. Mol. Cell Neurosci. 1997;9:194–206. doi: 10.1006/mcne.1997.0614. [DOI] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J. Physiol. 1997;499(Pt 3):715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Sun F, Thomas LL, Offord J, MacCallum DK, Dawson DC, Hughes BA, Ernst SA. Molecular cloning and expression of an inwardly rectifying K+ channel from bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2000;41:2936–2944. [PubMed] [Google Scholar]
- Yang D, Swaminathan A, Hughes BA. Expression of inwardly rectifying potassium channel subtypes in native bovine retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2002;44 doi: 10.1167/iovs.02-1189. ARVO E-Abstract 4568. [DOI] [PubMed] [Google Scholar]
- Yang D, Hughes BA. Molecular diversity of inwardly rectifying potassium channels in native human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003;44 doi: 10.1167/iovs.02-1189. ARVO E-Abstract 3451. [DOI] [PubMed] [Google Scholar]
- Yang D, Pan A, Swaminathan A, Kumar G, Hughes BA. Expression and localization of the inwardly rectifying potassium channel Kir7.1 in native bovine retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003a;44:3178–3185. doi: 10.1167/iovs.02-1189. [DOI] [PubMed] [Google Scholar]
- Yang D, MacCallum DK, Ernst SA, Hughes BA. Expression of the inwardly rectifying K+ channel Kir2.1 in native bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44(8):3511–3519. doi: 10.1167/iovs.02-1306. [DOI] [PubMed] [Google Scholar]
- Yang D, Swaminathan A, Zhang X, Hughes BA. Expression of Kir7.1 and a novel Kir7.1 splice variant in native human retinal pigment epithelium. Exp. Eye Res. 2008;86:81–91. doi: 10.1016/j.exer.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Shimura M, Hughes BA. Regulation of inwardly rectifying K+ channels in retinal pigment epithelial cells by intracellular pH. J. Physiol. 2003;549:429–438. doi: 10.1113/jphysiol.2003.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Kimura Y, Inui M. Effects of phospholipids on the oligomeric state of phospholamban of the cardiac sarcoplasmic reticulum. Circ. J. 2005;69:1116–1123. doi: 10.1253/circj.69.1116. [DOI] [PubMed] [Google Scholar]
- Zobel C, Cho HC, Nguyen TT, Pekhletski R, Diaz RJ, Wilson GJ, Backx PH. Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2. J. Physiol. 2003;550:365–372. doi: 10.1113/jphysiol.2002.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]