Abstract
The malaria parasite, Plasmodium falciparum, is unable to synthesize the purine ring de novo and is therefore wholly dependent upon purine salvage from the host for survival. Previous studies have indicated that a P. falciparum strain in which the purine transporter PfNT1 had been disrupted was unable to grow on physiological concentrations of adenosine, inosine and hypoxanthine. We have now used an episomally complemented pfnt1Δ knockout parasite strain to confirm genetically the functional role of PfNT1 in P. falciparum purine uptake and utilization. Episomal complementation by PfNT1 restored the ability of pfnt1Δ parasites to transport and utilize adenosine, inosine and hypoxanthine as purine sources. The ability of wild-type and pfnt1Δ knockout parasites to transport and utilize the other physiologically relevant purines adenine, guanine, guanosine and xanthine was also examined. Unlike wild-type and complemented P. falciparum parasites, pfnt1Δ parasites could not proliferate on guanine, guanosine or xanthine as purine sources, and no significant transport of these substrates could be detected in isolated parasites. Interestingly, whereas isolated pfnt1Δ parasites were still capable of adenine transport, these parasites grew only when adenine was provided at high, non-physiological concentrations. Taken together these results demonstrate that, in addition to hypoxanthine, inosine and adenosine, PfNT1 is essential for the transport and utilization of xanthine, guanine and guanosine.
Keywords: Plasmodium falciparum, malaria, PfNT1, purine, transport
1. INTRODUCTION
More than three billion people live at risk of contracting malaria, a disease caused by intra-erythrocytic protozoan parasites of the genus Plasmodium [1, 2]. Four species of Plasmodium are responsible for the majority of human malaria infections (P. falciparum, P. vivax, P. ovale and P. malariae), and cases of P. knowlesi infection in humans have recently been reported [3]. P. falciparum is the most lethal species, causing more than one million deaths annually [4]. With no effective vaccine available and drug resistance on the rise, the need for new antimalarial therapies is critical.
Protozoan parasites, unlike the human host, are incapable of purine biosynthesis and so are reliant upon the uptake of purines from their environment. As a consequence of this nutritional deficiency, each genus of parasite has evolved a unique complement of purine permeases and salvage enzymes that enables it to scavenge host purines effectively [5]. The first step in purine acquisition by P. falciparum is the translocation of host purines into the parasite, a process that fulfills an obligatory nutritional function and, as a result, offers potential targets for pharmacologic intervention.
The P. falciparum genome sequencing project uncovered four genes (PfNT1, PfNT2, PfNT3, and PfNT4) that harbor the majority of the signature sequences and topological features of the equilibrative nucleoside transporter family [6–8]. Of these, only PfNT1 has been functionally characterized at the molecular level [9, 10]. Immunofluorescence and immunoelectron microscopy analyses using antibodies directed toward this protein’s NH2-terminus revealed that PfNT1 is localized to the parasite plasma membrane [11]. Furthermore, western blot analysis demonstrated that PfNT1 is expressed throughout the intraerythrocytic life cycle but is maximally expressed prior to parasite schizogony, at the peak of nucleic acid synthesis and utilization [11]. Expression studies in Xenopus laevis oocytes established that PfNT1 exhibits broad ligand specificity for nucleosides and nucleobases [9, 10]. Studies by Downie and colleagues have shown that the transport properties of nucleosides and nucleobases across the parasite plasma membrane match closely those of PfNT1 expressed in Xenopus oocytes [12, 13].
Initial evidence for the functional importance of PfNT1 in purine metabolism was achieved by creating a pfnt1Δ null mutant in which the PfNT1 locus was disrupted by gene replacement [14]. Growth of this strain was only permissible on high, non-physiological concentrations of adenosine, inosine and hypoxanthine (presumably at high concentrations purines are able to cross the parasite plasma membrane by simple diffusion) [14], indicating that PfNT1 plays a key role in the utilization of host adenosine, inosine, and hypoxanthine as purine sources. The role PfNT1 plays in the transport and utilization of the other naturally occurring purines xanthine, guanine, guanosine and adenine has not yet been investigated.
We have now extended our analysis of purine transport to other physiologically relevant purines and demonstrated that PfNT1 is essential for parasite growth on guanine, guanosine, xanthine and adenine.
2. METHODS
2.1. Construction of the pfnt1Δ+PfNT1 complemented strain
To generate the pfnt1Δ+PfNT1 complemented strain, the open reading frame of PfNT1 was amplified by PCR from P. falciparum cDNA using the forward primer 5’-CCGCTCGAGATGAGTACCGGTAAAGAGTCATCT-3’ (XhoI site in bold, start codon underlined) and the reverse primer 5’-CCGCTCGAGTTATTGTGTTACATCGATGGGTGG-3’ (XhoI site in bold, stop codon underlined), digested and cloned into the XhoI site of the pHC1 vector [15], which encodes the P. falciparum CAM1 promoter and HSP86 terminator. Clones were selected and used to transform the pfnt1Δ strain using standard protocols [16]. Transgenic parasites harboring the pfnt1Δ+PfNT1 were selected by addition of 60 nM pyrimethamine to the culture medium and characterized by PCR to demonstrate both the loss of the PfNT1 chromosomal locus and the presence of the episome.
2.2. Microscopy
The intraerythrocytic development of P. falciparum was monitored by light microscopy on Giemsa-stained smears. Localization of PfNT1 in wild-type (3D7), pfnt1Δ and pfnt1Δ+PfNT1 parasites was determined by immunofluorescence as previously described [14]. Coverslips containing P. falciparum-infected erythrocyts were fixed and incubated for 1 h at room temperature with rabbit anti-PfNT1 polyclonal antibodies (diluted 1:500) and mouse anti-Band3 monoclonal antibody (1:500). Nuclei were stained by incubating the coverslips in phosphate buffered saline containing 3 µg/ml Hoechst 33258 (Molecular Probes) for 5 min at room temperature. The coverslips were then washed and mounted on slides with Prolong Antifade (Invitrogen), and images were analyzed by high-resolution fluorescence using deconvolution protocols. Microscopy was performed using an inverted microscope (Eclipse TE2000-E; Nikon) and filter 96320/HYQ (excitation 480 – 440 nm/emission 440 nm) for FITC, filter 96312/G2EC (excitation 540 – 525 nm/emission 620 – 660 nm) for rhodamine, and filter 96310/UV2EC (excitation 360–340 nm/emission 460–450 nm) for DAPI.
2.3. Parasite lactate dehydrogenase assay
The parasite-specific lactate dehydrogenase assay was performed in 96-well plates as described [17–19], 48 hours after synchronized, ring-stage infected red blood cells were added to RPMI containing increasing concentrations of purines. For each reaction, 20 µl of infected red blood cells were mixed with 10 µl of 1 mg/ml diaphorase, 10 µl of 1 mg/ml nitroblue tetrozolium and 100 µl of malstat reagent [17–19]. Parasite viability is indicated by the production of a purple color.
2.4. Transport assays in saponin-isolated parasites
Synchronized, mature trophozoite-stage (36 – 40 h post-invasion) parasites were ‘isolated’ from their host erythrocytes by treatment with saponin as described elsewhere [20]. Following saponin treatment, parasites were washed at least three times in a HEPES-buffered saline (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 20 mM glucose and 25 mM HEPES, pH 7.1). In order to distinguish between the transport and subsequent metabolism of radiolabeled purines, all experiments were carried out on parasites that had been depleted of their ATP content by incubation in a glucose free HEPES buffered saline (135 mM NaCl, 5 mM KCl, 1 mM MgCl2 and 25 mM HEPES, pH 7.1) followed by incubation at 37 °C for 15 min [21]. Parasites are wholly reliant on glycolysis for ATP synthesis [22]. Transport measurements were carried out essentially as reported previously [12, 13, 20], except that all experiments were carried out at room temperature (in order to allow measurable choline uptake, which acted as a control in all transport experiments). Briefly, uptake of radiolabelled solute was initiated by addition of a 200 µL volume of isolated parasites to an equal volume of radiolabeled substrate solution (at twice the intended final concentration) layered over a 200 µL dibutyl phthalate/dioctyl phthalate (5:4, v/v) inert oil mix. Uptake was stopped at pre-determined times by sedimenting the parasites below the oil layer using centrifugation in a rapid-acceleration microcentrifuge. The cell pellets were processed for scintillation counting as described previously [20], and estimates of the volume of extracellular fluid trapped in the cell pellets were made as described previously [12]. Uptake levels are expressed as ‘distribution ratios’ (i.e. the calculated intracellular concentration relative to the extracellular concentration of a particular solute).
2.5 Curve fitting
All curves were fitted using SigmaPlot version 7.0. For all time-course experiments, the curves were fitted to the full five data point, three minute time-course. To aid clarity the 3 min time points have been omitted from the scatter plots and are represented as bar graphs. Full time courses are provided as supplemental figures (Fig S1, Fig S2 and Fig S3)
3. RESULTS
3.1. Expression of the PfNT1 gene in the pfnt1Δ background rescues adenosine, inosine and hypoxanthine transport and nutritional defects of the knockout
To confirm that the defects in the utilization of adenosine, inosine and hypoxanthine in the pfnt1Δ strain [14] were not caused by secondary genetic alterations due to the loss of the PfNT1 chromosomal locus, a complemented strain (pfnt1Δ+PfNT1) was constructed in which the PfNT1 gene was expressed episomally within the pfnt1Δ genetic background. To generate the pfnt1Δ+PfNT1 strain, the PfNT1 ORF was cloned into the pHC1 vector, which encompasses the Toxoplasma gondii DHFR-TS marker conferring resistance to pyrimethamine [23]. In the resulting pHC1-PfNT1 vector, PfNT1 expression is under the regulatory control of the P. falciparum CAM1 promoter and HSP86 terminator (Fig 1A). This vector was then transfected into the pfnt1Δ knockout and parasites resistant to both pyrimethamine and blasticidin (by virtue of the replacement of the PfNT1 chromosomal locus with the BSD cassette) were selected. PfNT1 expression in the pfnt1Δ+PfNT1 strain was confirmed by reverse transcriptase PCR (Fig 1B) and immunofluorescence using affinity-purified anti-PfNT1 antibodies [11] (Fig 1C).
Figure 1.
Construction of a pfnt1Δ+PfNT1 complemented strain. (A) Map of the pHC1-PfNT1 vector used to transfect the pfnt1Δ knockout to create the complemented pfnt1Δ+PfNT1 strain. (B) Reverse transcriptase PCR analysis on wild-type, pfnt1Δ and pfnt1Δ+PfNT1 RNA using primers specific to PfNT1 or the P. falciparum phosphoethanolamine methyltransferase gene (PfPMT; used as a control). (C) Immunofluorescence microscopy of red blood cells infected with wild-type, pfnt1Δ or pfnt1Δ+PfNT1 schizont stage parasites. PfNT1 is visualized in green, Band-3 in red and nuclei in blue.
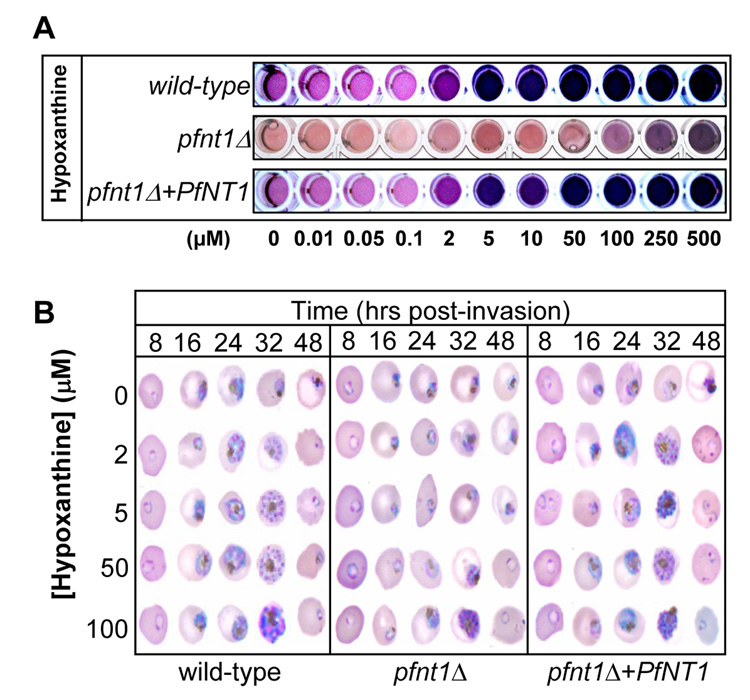
To determine whether the episomal expression of PfNT1 rescues the growth defects of the pfnt1Δ strain, the nutritional requirements of wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites were assessed by examining parasite growth when adenosine, inosine, and hypoxanthine were supplied as sole purine sources at concentrations ranging from 0 to 500 µM using the parasite-specific lactate dehydrogenase growth assay (Fig 2A) [17–19]. Whereas the pfnt1Δ strain was capable of growth only when hypoxanthine was present as a sole purine source at concentrations above 50 µM, wild-type and pfnt1Δ+PfNT1 strains exhibited robust growth when hypoxanthine was present as a sole purine source at concentrations as low as 2 µM (Fig 2A). Similar results were obtained with adenosine and inosine (data not shown). Giemsa-stained smears of parasite cultures were used to monitor development and life-cycle progression of each strain at different hypoxanthine concentrations (Fig 2B). Whereas wild-type and pfnt1Δ+PfNT1 parasites proceeded through the intraerythrocytic development cycle at hypoxanthine concentrations as low as 2 µM, the pfnt1Δ chromosomal knockout required 50–100 µM hypoxanthine in order to complete the intraerythrocytic life cycle (Fig 2B). Similar results were observed for growth on adenosine and inosine as sole purine sources (data not shown).
Figure 2.
Growth of wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites in the presence of increasing concentrations of hypoxanthine. (A) The viability of parasites at different hypoxanthine concentrations was determined using the parasite-specific lactate dehydrogenase assay. The presence of parasite lactate dehydrogenase, and hence viable cells, is indicated by the production of a purple color. The observed color differences between pfnt1Δ and wild-type/complement wells when purine concentration was below 2 µM are likely due to the presence of residual amounts of purine compounds in the erythrocytes, allowing limited parasite viability in the wild-type and complemented strains, whereas this minimal purine concentration is not sufficient to enable the presence of viable pfnt1Δ cells. (B) Synchronized wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites grown at different concentrations of hypoxanthine were collected at 8 h intervals, Giemsa-stained and evaluated by light microscopy. Similar results (for both assays) were obtained with inosine and adenosine (data not shown).
In order to determine if the episomal expression of PfNT1 can complement the transport defects seen in pfnt1Δ parasites, time-courses for the uptake of radiolabeled purines were carried out with ATP-depleted, saponin-isolated wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites. To test the viability of the ‘isolated’ ATP-depleted parasites, the ATP-independent, equilibrative transport of [14C]-choline, a compound that is not a PfNT1-ligand, was investigated. Previous work has indicated that the accumulation of [14C]-choline in energized parasites reflects a combination of transport and metabolic trapping (i.e. phosphorylation of choline by choline kinase) [24]. Depleting cells of their ATP content inhibits this metabolic trapping (as the phosphorylation of choline is energy dependent) and results in choline reaching a distribution ratio of 1 (indicating equal concentrations of choline inside and outside the cell) [24]. Consistent with previously published results [24, 25], ATP-replete wild-type and pfnt1Δ cells accumulated [14C]-choline in a linear fashion, reaching a distribution ratio (i.e. the concentration of a particular substrate inside the cell relative to that outside the cell) of ~20 after 10 min. In contrast, when cells from both strains were depleted of their ATP content, [14C]-choline levels reached a distribution ratio of ~1 (indicating equal concentrations inside and outside the cell) over a 30 s time period, a ratio that was maintained over the 10 min time course of the experiment (Fig. 3A). The transport of [14C]-choline was therefore used as a control for cell viability and ATP depletion in all subsequent purine transport experiments.
Figure 3.
Transport of radiolabeled substrates by P. falciparum parasites isolated from their host cells by saponin permeabilization of the erythrocyte and parasitophorous vacuole membranes. (A) Uptake of [14C]-choline by ATP-replete (squares, solid lines) or ATP-depleted (circles, dashed lines) wild-type (closed symbols) or pfnt1Δ (open symbols) parasites. Choline was present at an extracellular concentration of 1 µM. Uptake of the purines [3H]-adenosine (B); [3H]-inosine (C) and [3H]-hypoxanthine (D) by wild-type (closed circles), pfnt1Δ (open circles) and pfnt1Δ+PfNT1 (closed triangles) parasites that had been depleted of their ATP content. The uptake of each purine substrate at 180 s by wild-type (black bars), pfnt1Δ (grey bars) and pfnt1Δ+PfNT1 (white bars) parasites is shown in (E). In each case, the relevant unlabelled substrate was present at an extracellular concentration of 10 µM. Similar results were observed for pfnt1Δ parasites when unlabelled substrate was present at 0.1 µM (data not shown). The data for adenosine uptake into wild-type parasites and all pfnt1Δ data are averaged from three or more independent experiments (shown ± SEM) each carried out in duplicate. Inosine and hypoxanthine data from wild-type parasites and all data from pfnt1Δ+PfNT1 parasites are averaged from two independent experiments (shown ± SD), each carried out in duplicate. Where not shown, error bars fall within the symbols. All uptake data were obtained from 180 s time courses. For clarity, we have shown the 180 s time point on a separate graph (E). The curves in panels B, C and D were fitted to the complete 180 s time course, which has been provided as supplemental data, Fig S1.
Time courses for the uptake of adenosine, inosine and hypoxanthine by isolated wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites depleted of their ATP content are shown in Fig. 3 (panels B – E; full timecourses are shown in supplemental data, Fig S1). As observed previously [12], [3H]-adenosine (Fig 3B) and [3H]-inosine (Fig 3C) were taken up rapidly by wild-type 3D7 parasites with each [3H]-nucleoside reaching a distribution ratio of 2 – 3 in the first 10 s when the relevant unlabelled nucleoside was present at an extracellular concentration of 10 µM. This level of accumulation continued to rise, such that after three min the level of [3H]-adenosine within wild-type cells had reached a distribution ratio of 7.1 (± 0.7, n=2), and that of [3H]-inosine had reached a distribution ratio of 5.7 (± 0.9, n=2) (Fig 3E). In contrast, however, knockout parasites exhibited no initial uptake of [3H]-adenosine (Fig 3B) or [3H]-inosine (Fig 3C), and uptake remained negligible even after 3 min (Fig 3E). Similarly, knockout parasites showed negligible uptake of [3H]-adenosine and [3H]-inosine when the relevant unlabelled nucleoside was present at an extracellular concentration of 0.1 µM (data not shown). Nucleoside transport capability was restored in pfnt1Δ+PfNT1 parasites (closed triangles). In each case, the [3H]-nucleoside reached a distribution ratio approximating 1 within the first 30 s after addition of the radiolabel to the cell suspension (Fig 3B & 3C), and a distribution ratio of ~2 after three min (Fig 3E).
Consistent with published results [13], [3H]-hypoxanthine was transported rapidly by isolated wild-type parasites. When unlabelled hypoxanthine was present at an extracellular concentration of 10 µM, [3H]-hypoxanthine reached a distribution ratio of 1 within the first 10 s (Fig 3D), and this distribution ratio was maintained over 3 min (Fig 3E). Under the same conditions, pfnt1Δ parasites exhibited no uptake of [3H]-hypoxanthine over the first 60 s (Fig 3D), and only slight uptake after 3 min, reaching a distribution ratio of 0.2 (± 0.1; n = 3). Similar results were obtained when unlabelled hypoxanthine was present at 1 µM and 0.1 µM (data not shown). The transport of [3H]-hypoxanthine by pfnt1Δ+PfNT1 parasites (Fig 3D) was similar to that obtained for the transport of adenosine and inosine by this strain, with [3H]-hypoxanthine reaching a distribution level of 1.3 (± 0.2, n = 2) over 30 s, and 1.9 (± 0.4, n = 2) after 3 min (Fig 3E). Interestingly, however, this level of hypoxanthine uptake was actually higher than that detected in the wild-type strain (Fig 3D). These results clearly indicate that the transport deficits in pfnt1Δ parasites can be complemented by episomal expression of PfNT1. As such, subsequent transport experiments compared purine uptake in wild-type and knockout strains only.
3.2. PfNT1 is essential for the utilization but not the transport of adenine
The abilities of wild-type, pfnt1Δ and pfnt1Δ+PfNT1 strains to undergo asexual reproduction in the presence of varying concentrations of adenine was assessed using parasite-specific lactate dehydrogenase growth assays. Adenine supported growth of wild-type and pfnt1Δ+PfNT1 parasites at concentrations as low as 2 µM when present as a sole purine source (Fig 4A), similar to the concentrations of hypoxanthine, adenosine and inosine necessary to support growth of these strains (Fig 2). The pfnt1Δ knockout, however, was unable to grow when adenine was present as a sole purine source at concentrations below 50 µM, although higher concentrations of adenine permitted pfnt1Δ growth and progression through the intraerythrocytic development cycle (Fig. 4A). These results suggest that either PfNT1 is essential for the transport of adenine into the parasite or that adenine is converted in the red blood cell cytoplasm into a substrate that requires PfNT1 for its transport across the parasite plasma membrane. In order to distinguish between these two possibilities, transport experiments to measure the uptake of [3H]-adenine by pfnt1Δ parasites were performed. The conditions used were similar to those described above for adenosine, inosine and hypoxanthine (Fig 3), and to those used previously for the characterization of adenine transport in isolated wild-type parasites [13]. As shown in Fig 4, [3H]-adenine was taken up by both wild-type and pfnt1Δ parasites when adenine was present at 1.0 µM (Fig 4B & 4C) or 0.1 µM (Fig 4D & 4E). The data are also represented as 3 min time courses in Fig S2 (supplemental data) At each concentration, the total amount of adenine taken up by pfnt1Δ parasites was similar to that seen for wild-type parasites. When unlabelled adenine was present at an extracellular concentration of 1 µM (Fig 4B), [3H]-adenine was taken up rapidly by both wild-type and pfnt1Δ parasites, reaching a distribution ratio close to 2 within 30 seconds of the addition of radiolabel. After 3 min (Fig 4C), [3H]-adenine had reached a distribution ratio of 2.1 (± 0.1; n=3) in wild-type cells and 1.9 (± 0.1; n=4) in pfnt1Δ cells. A difference in the rate of uptake of [3H]-adenine between wild-type and pfnt1Δ cells was observed when the concentration of extracellular unlabelled adenine was 0.1 µM (Fig 4D). Whereas wild-type parasites showed rapid uptake of [3H]-adenine, reaching a distribution ratio of ~ 3 within 30 s, the amount of [3H]-adenine within pfnt1Δ parasites did not reach this level until 60 s after the addition of radiolabel. Intracellular adenine concentrations were comparable between the two strains after 3 min (wild-type: 3.2 ± 0.7, n=3; pfnt1Δ: 3.4 ± 0.9, n=4; Fig 4E). These transport results imply that while PfNT1 does account for some adenine transport a second uptake process exists at the parasite plasma membrane, which in addition to PfNT1 can enable entry of adenine into the parasite.
Figure 4.
Utilization and transport of adenine. Parasite growth in the presence of increasing concentrations of adenine was measured using the parasite-specific lactate dehydrogenase assay (A). Transport of [3H]-adenine by ATP-depleted parasites was measured when unlabelled adenine was present at an extracellular concentration of 1 µM (B, C) and 0.1 µM (D, E). Time courses for the uptake of adenine by wild-type (closed circles) and pfnt1Δ (open circles) parasites are shown in panels B and D. The curves are fitted to the data from the full 180 s time course (shown as supplemental data, Fig S2), but for clarity the 180 s data point was omitted from the time course and are shown in panels C & E. Black bars represent uptake by wild-type parasites, and grey bars that of pfnt1Δ parasites. pfnt1Δ data are averaged from four independent experiments (shown ± SEM), each carried out in duplicate. Wild-type data are averaged from three independent experiments (shown ± SEM), each carried out in duplicate.
3.3. PfNT1 is required for xanthine, guanine and guanosine transport
Human serum contains measurable quantities of xanthine, guanine and guanosine [26], which could serve as potential purine sources for P. falciparum. To assess the role of PfNT1 in the transport of these physiologically relevant purines across the P. falciparum parasite plasma membrane, the transport and nutritional requirements for these 6-oxypurines was compared in wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites (Fig 5). Wild-type and pfnt1Δ+PfNT1 strains were able to grow when guanine, guanosine, and xanthine were present as sole purine sources at concentrations as low as 2 µM, while pfnt1Δ parasites were unable to complete the intraerythrocytic life cycle at concentrations of these ligands up to 50 µM (Fig. 5A and B). Interestingly, xanthine, guanine and guanosine were found to be toxic to wild-type parasites at concentrations higher than 50 µM (data not shown). Thus, whether higher concentrations of xanthine, guanine, or guanosine could meet the purine requirement of pfnt1Δ parasites could not be determined.
Figure 5.
Growth of wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites in the presence of increasing concentrations of guanine, guanosine and xanthine. (A) Parasite viability was assessed using the parasite-specific lactate dehydrogenase assay. (B) Synchronized wild-type, pfnt1Δ and pfnt1Δ+PfNT1 parasites were cultured in increasing concentrations of guanine, collected at eight hour intervals, Giemsa stained and evaluated by light microscopy. Similar results were obtained with guanosine and xanthine (data not shown).
To examine the involvement of PfNT1 in the transport of xanthine, guanine and guanosine across the parasite plasma membrane, time courses for the uptake of [3H]-xanthine, [3H]-guanine and [3H]-guanosine by isolated, ATP-depleted mature trophozoite-stage parasites were carried out (in the presence of 0.1 µM concentrations of the unlabelled substrate of interest). As shown in Fig 6A, wild-type cells transported xanthine rapidly, reaching a distribution ratio of 0.9 (± 0.2, n=2) within the first 10 sec, a level that was maintained for the 3 min duration of the time-course (Fig 6B). pfnt1Δ parasites, however, showed negligible uptake of xanthine over the first 60 s (Fig 6A), although measurable uptake of [3H]-xanthine was detected after 3 min, reaching a distribution ratio of 0.4 (± 0.1, n=2) (Fig 6B). This rate of uptake was much slower than that seen in wild-type cells and may represent simple diffusion of the compound over time. The data are shown as 3 min time-courses in the supplemental data, Fig S3A.
Figure 6.
Uptake of xanthine, guanine and guanosine by ATP-depleted, saponin-isolated wild-type (closed circles) and pfnt1Δ (open circles) P. falciparum parasites. Uptake of [3H]-xanthine (A, B); [3H]-guanine (C, D) and [3H]-guanosine (E, F) measured in the presence of a 0.1 µM concentration of the relevant unlabelled purine. Curves are fitted to the full 180 s time course, and in each case the 180 s data point has been shown on a separate graph to aid clarity and allow comparison to the choline control. The data are represented as a full time course in the supplemental data (Fig S3). Wild-type data are averaged from two independent experiments (shown ± SD), each carried out in duplicate. pfnt1Δ data are averaged from three independent experiments (shown ± SEM), each carried out in duplicate. Where not shown, error bars fall within the symbols.
[3H]-Guanine and [3H]-guanosine were also rapidly taken up by isolated wild-type cells, attaining distribution ratios of ~2 within the first 10 sec (Fig 6C & 6E; see also Fig S3B& S3C). Uptake continued over time with each radiolabeled purine compound attaining a distribution ratio of ~4 after 3 min (Fig 6D & 6F). In contrast, under the same conditions, pfnt1Δ parasites displayed slight uptake of guanine (0.3 ± 0.1; Fig 6C & 6D; similar to that seen for xanthine) and only negligible uptake of radiolabeled guanosine (Fig 6E, F). These results are consistent with PfNT1 being essential for the transport of the 6-oxypurines xanthine, guanine and guanosine across the P. falciparum plasma membrane.
4. DISCUSSION
It has recently been demonstrated that PfNT1 plays an essential function in the uptake of host hypoxanthine across the parasite plasma membrane [14]. Under physiological conditions of purine supply, transgenic pfnt1Δ parasites lacking PfNT1 activity display a conditionally lethal growth phenotype that could be circumvented by the provision of excess concentrations of hypoxanthine, inosine, or adenosine to the culture medium. We have now created and exploited a PfNT1 complemented strain, pfnt1Δ+PfNT1, to prove definitively that the transport and nutritional deficits observed in the pfnt1Δ strain were due to the deletion of PfNT1 rather than to any other chromosomal rearrangements resulting from the knockout of the PfNT1 locus. The pfnt1Δ+PfNT1 strain, like wild-type parasites, was able to grow on physiological concentrations of adenosine, inosine, hypoxanthine, adenine, xanthine, guanine and guanosine, thus complementing the PfNT1 genetic lesion in the pfnt1Δ knockout.
In order to ensure that the saponin-isolated, ATP depleted parasites used in uptake experiments were viable, time-courses for the uptake of [14C]-choline acted as a control in all experiments. The uptake of choline by isolated parasites is highly temperature dependent, with little uptake occurring at 0°C [25] or 4°C (this study, data not shown). As such, all transport experiments presented here were carried out at room temperature. This may account for the differences observed between the equilibration of adenine and adenosine seen previously for isolated, ATP-depleted cells [12, 13] and the accumulation seen here (all other conditions were identical). Performing the uptakes at this temperature also meant that we were unable to acquire data within the linear phase of uptake for these substrates, meaning that kinetic characterization of transport (where observed) was not feasible, as determination of initial rates of transport was not possible. The kinetics of such transport, however, have previously been studied in some detail for wild-type parasites [12, 13].
Transport studies in isolated parasites showed that pfnt1Δ+PfNT1 parasites, unlike pfnt1Δ parasites, were capable of efficient transport of adenosine, inosine and hypoxanthine. Adenosine and inosine reached lower concentrations in the complemented strain than they did in the wild-type strain, while complemented parasites took up slightly more hypoxanthine than wild-type parasites. These differences were minor and could be ascribed to the reintroduction of episomal PfNT1 and its expression from the CAM1 promoter in the complemented strain. The complemented strain showed similar levels of adenosine, inosine and hypoxanthine (where as hypoxanthine uptake was lower than adenosine or inosine in wild-type parasites). It is possible that the expression of purine salvage proteins is altered in pfnt1Δ parasites, and that this difference in maintained in the pfnt1Δ+PfNT1 strain. Overall, the data obtained here using the pfnt1Δ+PfNT1 parasites demonstrate that the purine transport and utilization defects seen in pfnt1Δ parasites are solely due to the loss of the PfNT1 gene.
In the current study, the characterization of the pfnt1Δ parasites has been extended to examine transport and utilization of other physiologically relevant purines, specifically adenine, xanthine, guanine and guanosine. While wild-type and pfnt1Δ+PfNT1 parasites were able to grow on adenine as a sole purine source at concentrations as low as 2 µM, pfnt1Δ parasites were able to grow on adenine only when this compound was provided at concentrations above 50 µM (Fig 4A). Intriguingly however, these parasites were still able to transport [3H]-adenine. At 0.1 µM adenine, the initial rate of transport of [3H]-adenine was somewhat slower in pfnt1Δ parasites than in wild type cells, consistent with a PfNT1-dependent component of adenine transport (Fig 4D). However, the levels of adenine uptake after 3 min were comparable (Fig 4). The observation of a PfNT1-dependent adenine transport component is consistent with work by Downie et al [13], while the presence of adenine transport process(es) in pfnt1Δ parasites suggests that adenine can also cross the parasite plasma membrane by an additional transport mechanism. The lack of growth of the pfnt1Δ strain at physiological concentrations of adenine (the plasma concentration of adenine is ~0.4 µM) could be explained if all adenine entering the erythrocyte is rapidly converted to those purines dependent upon PfNT1 for transport into the parasite, either in the erythrocyte cytosol or the parasitophorous vacuole, such that adenine never reaches the parasite plasma membrane (see model in Fig. 7). Alternatively, although some adenine may reach the parasite plasma membrane of the P. falciparum-infected erythrocyte and be transported into the parasite by a PfNT1-independent mechanism, but the parasite may not be able to utilize this compound. Available biochemical data and BLAST searches of the P. falciparum genome [7], suggest that the parasite lacks the enzymes capable of metabolizing adenine [27]. The erythrocyte, however, has the metabolic machinery to convert exogenously provided adenine to PfNT1 substrates. Adenine can be phosphoribosylated by adenine phosphoribosyltransferase to AMP, AMP can be deaminated by AMP deaminase to IMP, and IMP can be desphosphorylated to inosine, a PfNT1 substrate, by mammalian cells. Thus, it is plausible that the pfnt1Δ growth defect could be attributed to an inability of the mutant parasite to take up adenine metabolites such as inosine formed in the erythrocyte cytosol.
Figure 7.
Model of the pathways for purine uptake in P. falciparum. Hypoxanthine (HXT); adenosine (ADO); inosine (INO); adenine (ADE); xanthine (XAN); guanine (GUA), and guanosine (GUO) are transported by means of endogenous purine transporters and the NPP. Adenine could be phosphoribosylated to AMP in the RBC cytoplasm by APRT, converted to IMP via AMP deaminase, dephosphorylated by an intracellular 5'-nucleotidase to inosine, and converted to hypoxanthine by PNP. The additional parasite plasma membrane permease(s) for the transport of adenine, represented by dotted lines, are unlikely to play an important role under physiological conditions. For clarity, the parasitophorous vacuole membrane has been omitted from this model. PNP, purine nucleoside phosphorylase; ADA, adenosine deaminase; PV, parasitophorous vacuole; RBCM, RBC membrane.
Wild-type and complemented parasites were capable of growth on guanine, xanthine or guanosine as a sole purine source, even at concentrations as low as 2 µM. Conversely, pfnt1Δ parasites were unable to utilize these 6-oxypurines as a purine source at concentrations below 50 µM (Fig. 6), similar to results seen previously for adenosine, inosine and hypoxanthine [14]. Although we tested whether concentrations of guanine, xanthine, and guanosine between 50 and 1000 µM could rescue the growth defect of pfnt1Δ parasites, these high, non-physiological concentrations of these three purines inhibited growth of wild-type parasites (data not shown). Thus lack of growth of pfnt1Δ parasites at high concentrations of xanthine, guanine and guanosine cannot be imputed to a lack of functional rescue. Coupled with the impaired capacity of pfnt1Δ parasites to take up guanine, xanthine, and guanosine efficiently, the growth assays provide powerful genetic evidence that PfNT1 is the major route of entry of 6-oxypurines into the parasite.
In addition to PfNT1, the P. falciparum genome contains three putative transporters (PfNT2, PfNT3, and PfNT4) predicted to be equilibrative nucleoside transporters, suggesting that additional purine transport mechanisms exist in the parasite. While it is possible that these proteins may play a role in the transport of purines across the red blood cell membrane or across organelles within the parasite, the results accumulated thus far indicate that PfNT1 is the only purine transporter required for the uptake of physiologically relevant purine nucleosides and nucleobases across the parasite plasma membrane of intra-erythrocytic forms of P. falciparum. The roles of these transporters in the PfNT1-independent transport of adenine and in the biochemistry and physiology of other mammalian or insect stages of the P. falciparum lifecycle remain to be explored.
The data presented here for the uptake of adenosine, inosine, adenine, guanine and guanosine by wild-type parasites show that unlike choline (and xanthine), these substrates reached an intracellular concentration higher than the extracellular concentration. The conditions used here (glucose- and ATP-depletion) were chosen in order to prevent phosphorylation (as shown for choline) and phosphoribosylation of the transported substrates. While it is possible that the accumulation is due to slow metabolism as a result of residual ATP content in the glucose-deprived cells, the fact that choline consistently reached distribution ratios of ~1 over a 3 min time course, rather than the distribution ratio of ~5 in ATP-replete cells would argue against this. Additionally, the observation that guanine is accumulated whereas xanthine is not suggests that this accumulation is not HGXPRT dependent, as both of these compounds are substrates of this enzyme. It is unlikely that the accumulation is due to active transport by PfNT1, as ATP is required to maintain H+ and Na+ gradients, and not all PfNT1 substrates are accumulated under these conditions. Additionally, multiple studies have shown that PfNT1 transport is neither H+ or Na+ dependent [9, 10]. This suggests that upon their entry into the parasite, some of the transported substrates may be rapidly sequestered in one or more organelles of the parasite. Future studies aimed at the localization and characterization of PfNT2, PfNT3, and PfNT4 could help unravel this mechanism of accumulation, and help explain the need for additional transporters during the parasite’s intraerythrocytic life cycle.
The transport results described here for adenosine and inosine differ slightly from our previously reported data, which showed residual uptake of these substrates in energized, isolated pfnt1Δ parasites [14]. The different methods used for parasite isolation and radiolabel uptake experiments as well as the energy status of the isolated parasites could account for some of the discrepancies seen. Nevertheless, the combined results of purine transport and utilization in pfnt1Δ parasites support the essential role of PfNT1 in the transport and utilization of all purines.
Recently, Quashie and colleagues [28] have suggested that there are four separate purine transport activities, and report the PfNT1-independent uptake of several purines. The data we have reported here does agree on one point (the existence of a PfNT1-independent adenine transport mechanism), however we did not observe any other PfNT1-independent purine transport mechanisms. We believe that the discrepancies could be due to differences in experimental methods as well as a failure on their part to distinguish between transport and metabolism.
In summary, the current study provides genetic evidence for the essential role of PfNT1 in the transport and utilization of xanthine, guanine and guanosine in P. falciparum. Future studies will focus on therapeutic strategies to target this transporter, either to block purine uptake into the parasite or as a route for the specific delivery of new antimalarial drugs to the parasite cytoplasm.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Harriet Zawistowski (General Clinical Research Center, University of Connecticut Health Center) for her technical help. This research was supported by grant AI51507 from the National Institute of Allergy and Infectious Disease (to C.B.M and B.U.); grant PR033005 from the Department of Defense to C.B.M and the Burroughs Wellcome fund (to C.B.M). C.B.M is a recipient of the Burroughs Wellcome Award (1006267), Investigators of Pathogenesis of Infectious Diseases. This work was also supported in part by National Institutes of Health General Clinical Research Center Grant M01RR06192 (to the University of Connecticut Health Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 2.Guerra CA, Snow RW, Hay SI. Defining the global spatial limits of malaria transmission in 2005. Adv Parasitol. 2006;62:157–179. doi: 10.1016/S0065-308X(05)62005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 4.Geneva: World Health Organisation; World Malaria Report 2005. 2005
- 5.Carter N, Rager N, Ullman B. Purine and Pyrimidine Transport and Metabolism. In: Marr TN JJ, Komuniecki R, editors. Molecular and Medical Parasitology. London: Academic Press Limited; 2003. pp. 197–223. [Google Scholar]
- 6.Baldwin SA, McConkey GA, Cass CE, Young JD. Nucleoside transport as a potential target for chemotherapy in malaria. Curr Pharm Des. 2007;13:569–580. doi: 10.2174/138161207780162845. [DOI] [PubMed] [Google Scholar]
- 7.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin RE, Henry RI, Abbey JL, Clements JD, Kirk K. The 'permeome' of the malaria parasite: an overview of the membrane transport proteins of Plasmodium falciparum. Genome Biol. 2005;6:R26. doi: 10.1186/gb-2005-6-3-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter NS, Ben Mamoun C, Liu W, Silva EO, Landfear SM, Goldberg DE, Ullman B. Isolation and functional characterization of the PfNT1 nucleoside transporter gene from Plasmodium falciparum. J Biol Chem. 2000;275:10683–10691. doi: 10.1074/jbc.275.14.10683. [DOI] [PubMed] [Google Scholar]
- 10.Parker MD, Hyde RJ, Yao SY, McRobert L, Cass CE, Young JD, McConkey GA, Baldwin SA. Identification of a nucleoside/nucleobase transporter from Plasmodium falciparum, a novel target for anti-malarial chemotherapy. Biochem J. 2000;349:67–75. doi: 10.1042/0264-6021:3490067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rager N, Mamoun CB, Carter NS, Goldberg DE, Ullman B. Localization of the Plasmodium falciparum PfNT1 nucleoside transporter to the parasite plasma membrane. J Biol Chem. 2001;276:41095–41099. doi: 10.1074/jbc.M107037200. [DOI] [PubMed] [Google Scholar]
- 12.Downie MJ, Saliba KJ, Howitt SM, Broer S, Kirk K. Transport of nucleosides across the Plasmodium falciparum parasite plasma membrane has characteristics of PfENT1. Mol Microbiol. 2006;60:738–748. doi: 10.1111/j.1365-2958.2006.05125.x. [DOI] [PubMed] [Google Scholar]
- 13.Downie MJ, Saliba KJ, Broer S, Howitt SM, Kirk K. Purine nucleobase transport in the intraerythrocytic malaria parasite. Int J Parasitol. 2008;38:203–209. doi: 10.1016/j.ijpara.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.El Bissati K, Zufferey R, Witola WH, Carter NS, Ullman B, Ben Mamoun C. The plasma membrane permease PfNT1 is essential for purine salvage in the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 2006;103:9286–9291. doi: 10.1073/pnas.0602590103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabb BS, Triglia T, Waterkeyn JG, Cowman AF. Stable transgene expression in Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:131–144. doi: 10.1016/s0166-6851(97)00143-6. [DOI] [PubMed] [Google Scholar]
- 16.Fidock DA, Nomura T, Wellems TE. Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol Pharmacol. 1998;54:1140–1147. doi: 10.1124/mol.54.6.1140. [DOI] [PubMed] [Google Scholar]
- 17.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 18.Makler MT, Piper RC, Milhous WK. Lactate dehydrogenase and the diagnosis of malaria. Parasitol Today. 1998;14:376–377. doi: 10.1016/s0169-4758(98)01284-8. [DOI] [PubMed] [Google Scholar]
- 19.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinrichs DJ. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 20.Saliba KJ, Horner HA, Kirk K. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J Biol Chem. 1998;273:10190–10195. doi: 10.1074/jbc.273.17.10190. [DOI] [PubMed] [Google Scholar]
- 21.Saliba KJ, Kirk K. pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H(+) extrusion via a v-type h(+)-atpase. J Biol Chem. 1999;274:33213–33219. doi: 10.1074/jbc.274.47.33213. [DOI] [PubMed] [Google Scholar]
- 22.Sherman IW. Malaria parasite biology pathogenesis and protection. ASM Press; 1998. Carbohydrate metabolism of asexual stages; pp. 135–143. [Google Scholar]
- 23.Crabb BS, Cowman AF. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehane AM, Saliba KJ, Allen RJ, Kirk K. Choline uptake into the malaria parasite is energized by the membrane potential. Biochem Biophys Res Commun. 2004;320:311–317. doi: 10.1016/j.bbrc.2004.05.164. [DOI] [PubMed] [Google Scholar]
- 25.Biagini GA, Pasini EM, Hughes R, De Koning HP, Vial HJ, O'Neill PM, Ward SA, Bray PG. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood. 2004;104:3372–3377. doi: 10.1182/blood-2004-03-1084. [DOI] [PubMed] [Google Scholar]
- 26.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary K, Darling JA, Fohl LM, Sullivan WJ, Jr, Donald RG, Pfefferkorn ER, Ullman B, Roos DS. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2004;279:31221–31227. doi: 10.1074/jbc.M404232200. [DOI] [PubMed] [Google Scholar]
- 28.Quashie NB, Dorin-Semblat D, Bray PG, Biagini G, Doerig C, Ranford-Cartwright LC, De Koning HP. A comprehensive model of purine uptake by the malaria parasite Plasmodium falciparum: identification of four purine transport activities in intraerythrocytic parasites. Biochem J. 2008;411:287–295. doi: 10.1042/BJ20071460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.