Abstract
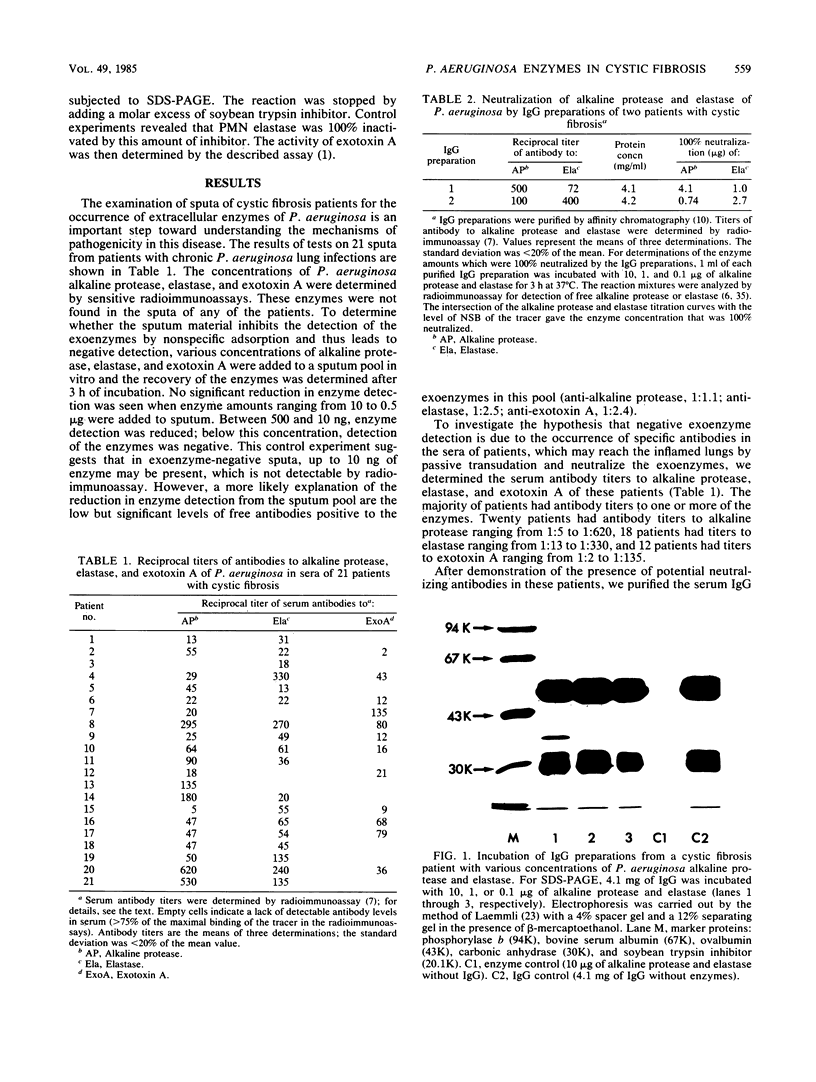
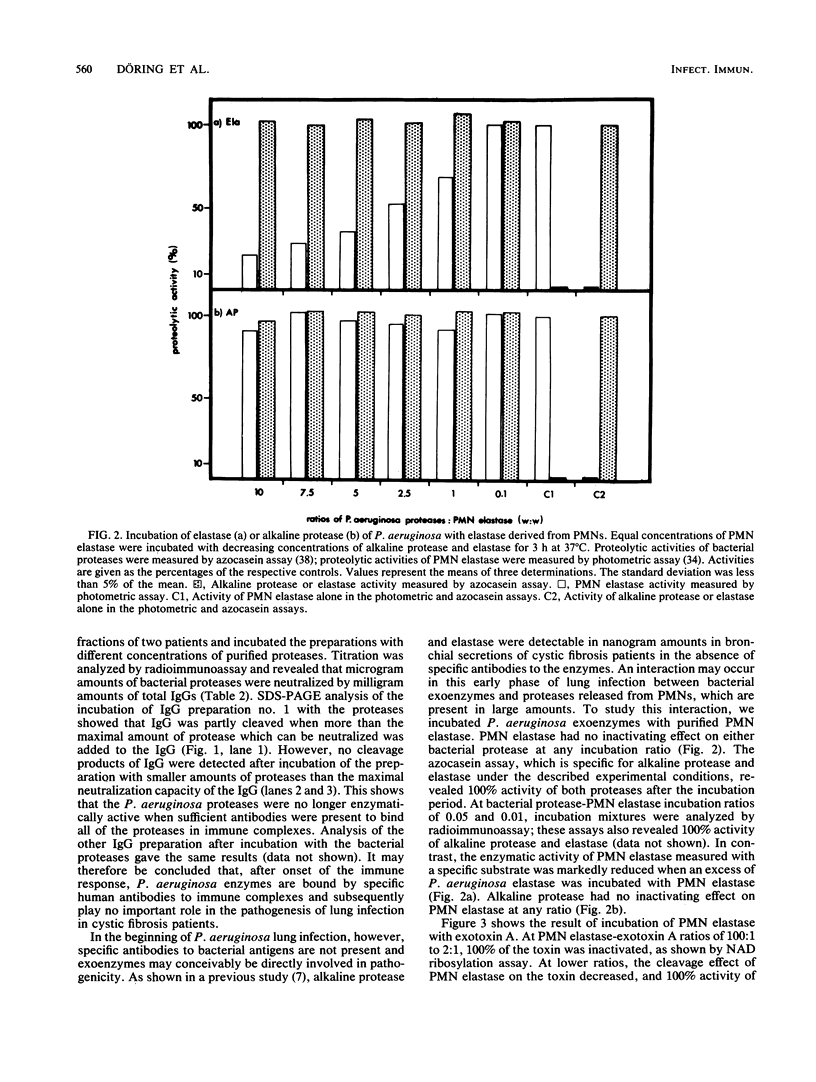
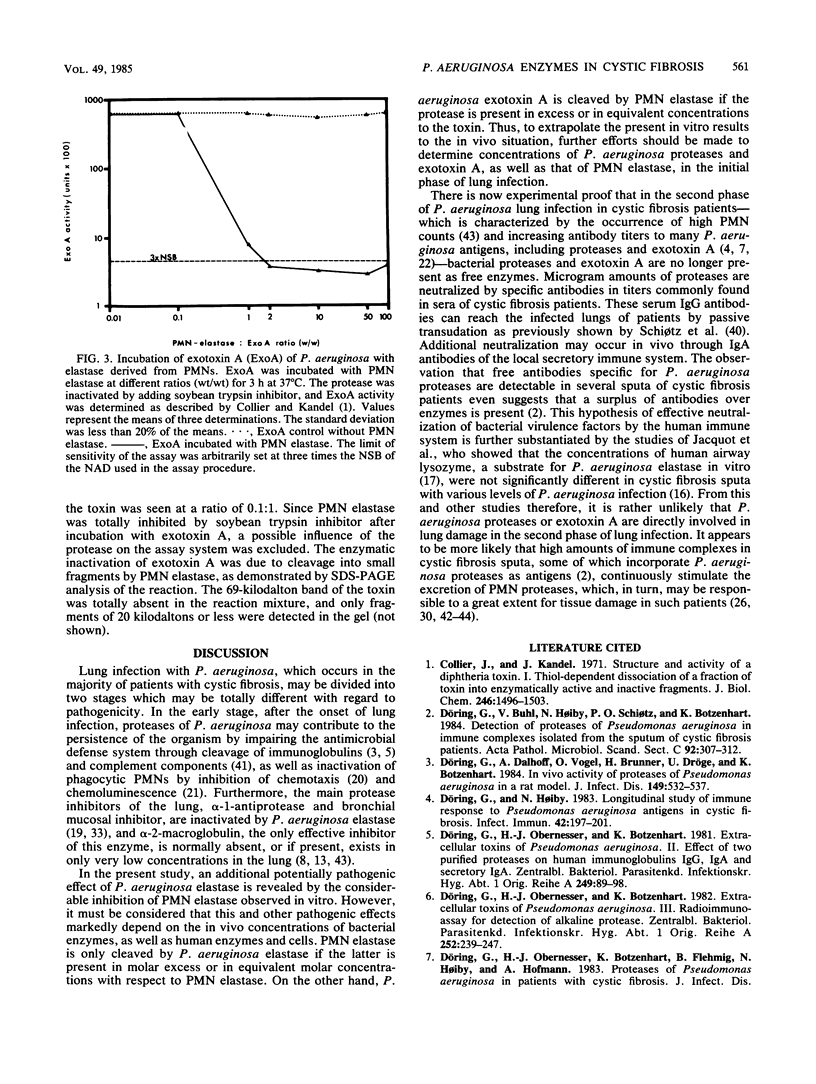
We investigated the role of Pseudomonas aeruginosa exoenzymes in cystic fibrosis lung infection in the presence and absence of specific serum antibodies. In sputa of 21 cystic fibrosis patients, concentrations of P. aeruginosa proteases and exotoxin A were determined by sensitive radioimmunoassays. In all sputa, detection of exoenzymes was negative (less than or equal to 10 ng). Positive serum antibody titers to bacterial exoenzymes were found in the majority of patients. Purified immunoglobulin G (IgG) preparations from the sera of two patients revealing specific antibody titers to the bacterial proteases neutralized these enzymes at ratios of 1,000:1 to 5,600:1 (wt/wt). Above the neutralizing capacity of IgG, proteases caused cleavage of IgG; below that level, no enzymatic activity was observed. In vitro incubation of P. aeruginosa elastase, alkaline protease, or exotoxin A with elastase derived from polymorphonuclear leukocytes showed that polymorphonuclear leukocyte elastase: (i) was cleaved by bacterial elastase, (ii) was not inactivated by alkaline protease, and (iii) inactivated exotoxin A. The results suggest that soon after the onset of P. aeruginosa lung infection in cystic fibrosis patients, bacterial proteases, but not exotoxin A, become important virulence factors. The results also suggest that exoenzymes do not directly contribute to lung damage after immune response to bacterial antigens has begun.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Döring G., Buhl V., Høiby N., Schiøtz P. O., Botzenhart K. Detection of proteases of Pseudomonas aeruginosa in immune complexes isolated from sputum of cystic fibrosis patients. Acta Pathol Microbiol Immunol Scand C. 1984 Oct;92(5):307–312. doi: 10.1111/j.1699-0463.1984.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Döring G., Dalhoff A., Vogel O., Brunner H., Dröge U., Botzenhart K. In vivo activity of proteases of Pseudomonas aeruginosa in a rat model. J Infect Dis. 1984 Apr;149(4):532–537. doi: 10.1093/infdis/149.4.532. [DOI] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extracellular toxins of Pseudomonas aeruginosa. III. Radioimmunoassay for detection of alkaline protease. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):239–247. [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Hoiby N., Flensborg E. W., Beck B., Friis B., Jacobsen S. V., Jacobsen L. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. Scand J Respir Dis. 1977 Apr;58(2):65–79. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Jacquot J., Tournier J. M., Carmona T. G., Puchelle E., Chazalette J. P., Sadoul P. Protéines des sécrétions bronchiques dans la mucoviscidose. Role de l'infection. Bull Eur Physiopathol Respir. 1983 Sep-Oct;19(5):453–458. [PubMed] [Google Scholar]
- Jacquot J., Tournier J. M., Puchelle E. In vitro evidence that human airway lysozyme is cleaved and inactivated by Pseudomonas aeruginosa elastase and not by human leukocyte elastase. Infect Immun. 1985 Feb;47(2):555–560. doi: 10.1128/iai.47.2.555-560.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Carter-Hamm B., Dralle W. M. Inactivation of human bronchial mucosal proteinase inhibitor by Pseudomonas aeruginosa elastase. Am Rev Respir Dis. 1982 Dec;126(6):1070–1073. doi: 10.1164/arrd.1982.126.6.1070. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Döring G., Høiby N., Valerius N. H. Interaction of Pseudomonas aeruginosa alkaline protease and elastase with human polymorphonuclear leukocytes in vitro. Infect Immun. 1984 Jan;43(1):161–165. doi: 10.1128/iai.43.1.161-165.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Høiby N., Döring G., Valerius N. H. Pseudomonas aeruginosa exoproteases inhibit human neutrophil chemiluminescence. Infect Immun. 1984 Jun;44(3):587–591. doi: 10.1128/iai.44.3.587-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger J. D., Straus D. C., Hilton C. B., Bass J. A. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: Demonstration by radioimmunoassay. J Infect Dis. 1978 Jul;138(1):49–48. doi: 10.1093/infdis/138.1.49. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN J., KURNICK N. B. Proteolytic enzyme activity and the role of desoxyribose nucleic acid (DNA) in cystic fibrosis sputum. Pediatrics. 1963 Jun;31:1028–1032. [PubMed] [Google Scholar]
- LIEBERMAN J., TRIMMER B. M., KURNICK N. B. SUBSTRATE SPECIFICITY OF PROTEASE ACTIVITIES IN PURULENT SPUTUM. Lab Invest. 1965 Mar;14:249–257. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J., Kaneshiro W. Inhibition of leukocytic elastase from purulent sputum by alpha 1 -antitrypsin. J Lab Clin Med. 1972 Jul;80(1):88–101. [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martodam R. R., Baugh R. J., Twumasi D. Y., Liener I. E. A rapid procedure for the large scale purification of elastase and cathepsin G from human sputum. Prep Biochem. 1979;9(1):15–31. doi: 10.1080/00327487908061669. [DOI] [PubMed] [Google Scholar]
- May J. R., Herrick N. C., Thompson D. Bacterial infection in cystic fibrosis. Arch Dis Child. 1972 Dec;47(256):908–913. doi: 10.1136/adc.47.256.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Obernesser H. J., Döring G. Extracellular toxins of Pseudomonas aeruginosa. IV. Radioimmunoassay for detection of elastase. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):248–256. [PubMed] [Google Scholar]
- Pollack M., Callahan L. T., 3rd, Taylor N. S. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: evidence for in vivo toxin production during infection. Infect Immun. 1976 Oct;14(4):942–947. doi: 10.1128/iai.14.4.942-947.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C. F., Travis J. The degradation of human lung elastin by neutrophil proteinases. Biochim Biophys Acta. 1980 Jan 24;621(1):147–157. doi: 10.1016/0005-2795(80)90070-7. [DOI] [PubMed] [Google Scholar]
- Schiøtz P. O., Clemmensen I., Høiby N. Immunoglobulins and albumin in sputum from patients with cystic fibrosis. A study of protein stability and presence of proteases. Acta Pathol Microbiol Scand C. 1980 Oct;88(5):275–280. doi: 10.1111/j.1699-0463.1980.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Twumasi D. Y., Liener I. E. Proteases from purulent sputum. Purification and properties of the elastase and chymotrypsin-like enzymes. J Biol Chem. 1977 Mar 25;252(6):1917–1926. [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Everitt M. T., Neurath H., Woodbury R. G., Powers J. C. Substrate specificity of two chymotrypsin-like proteases from rat mast cells. Studies with peptide 4-nitroanilides and comparison with cathepsin G. Biochemistry. 1980 Dec 9;19(25):5799–5804. doi: 10.1021/bi00566a021. [DOI] [PubMed] [Google Scholar]


