Abstract
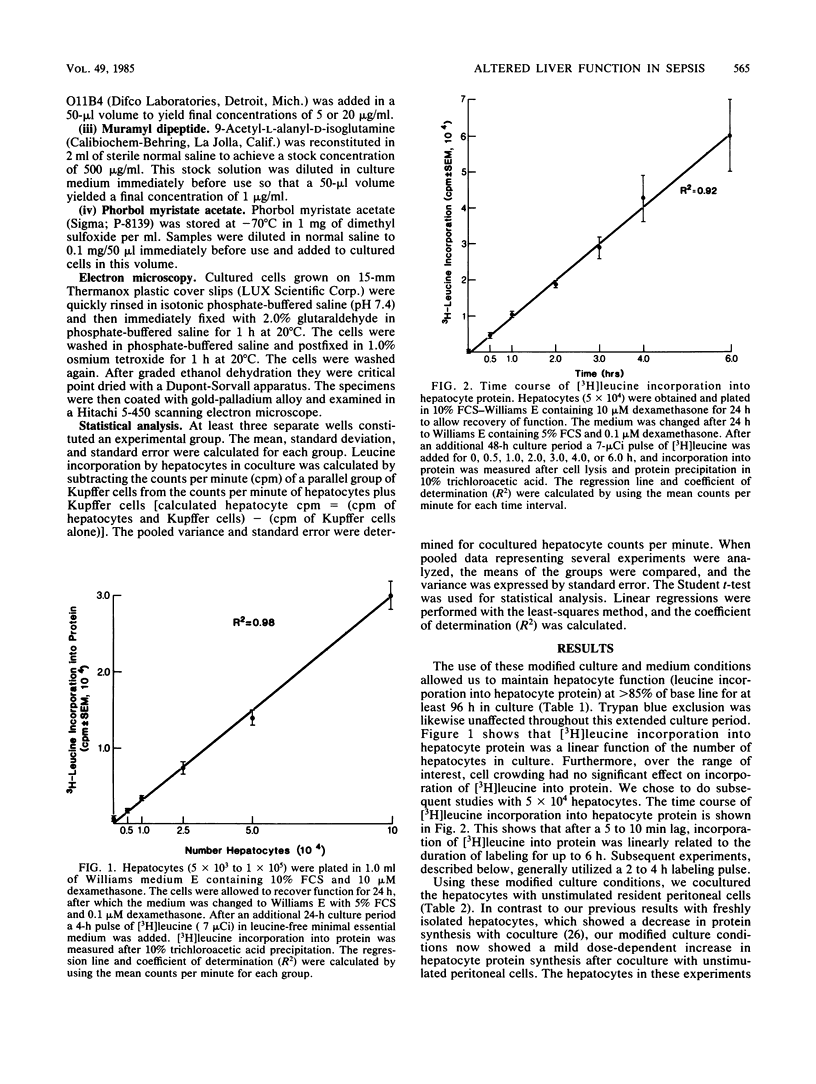
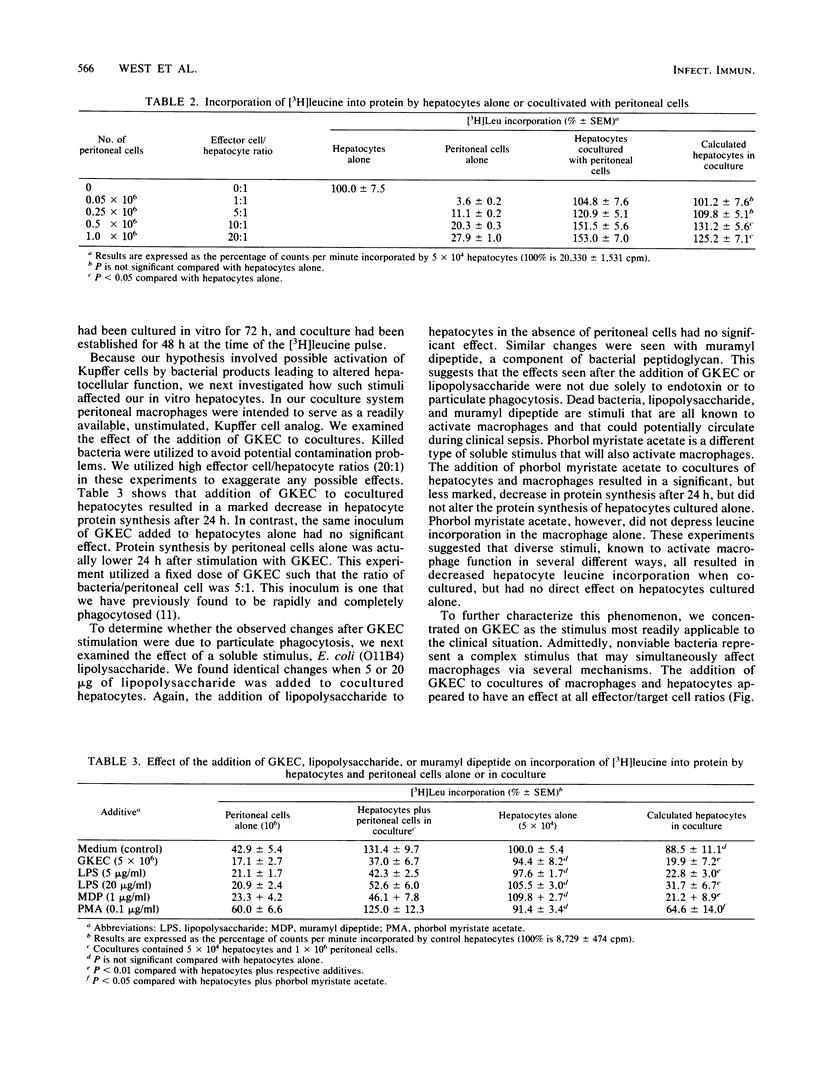
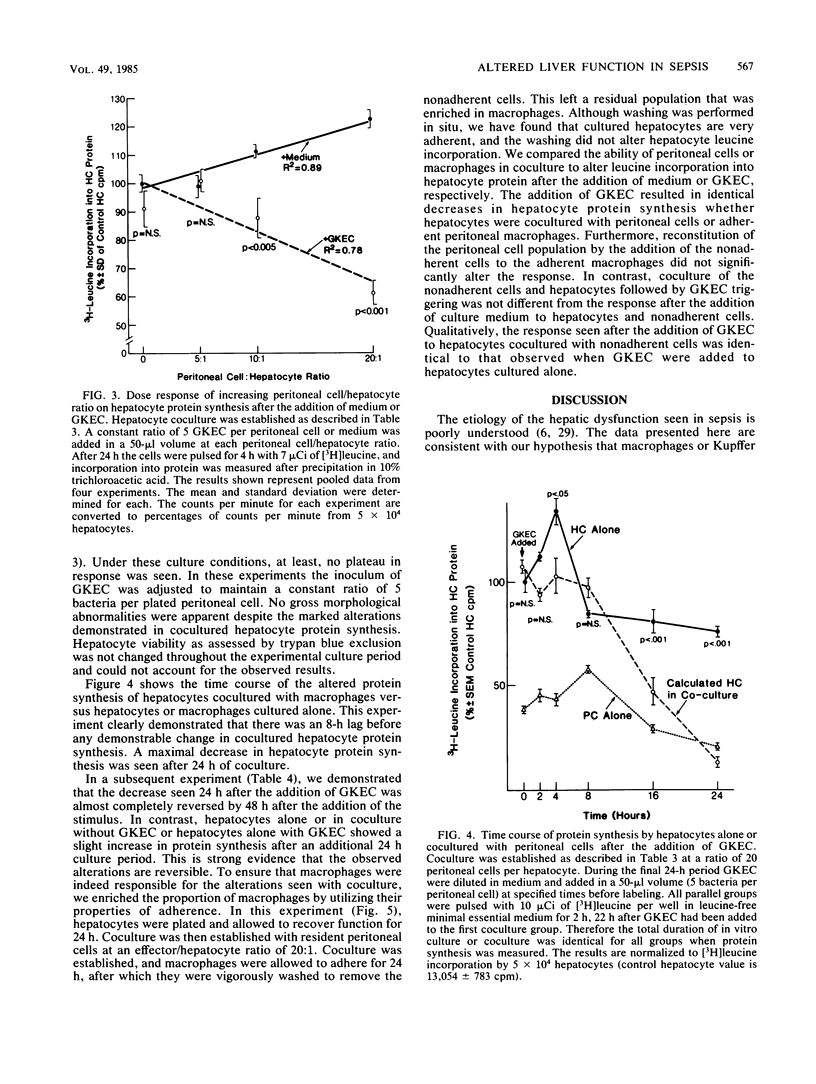
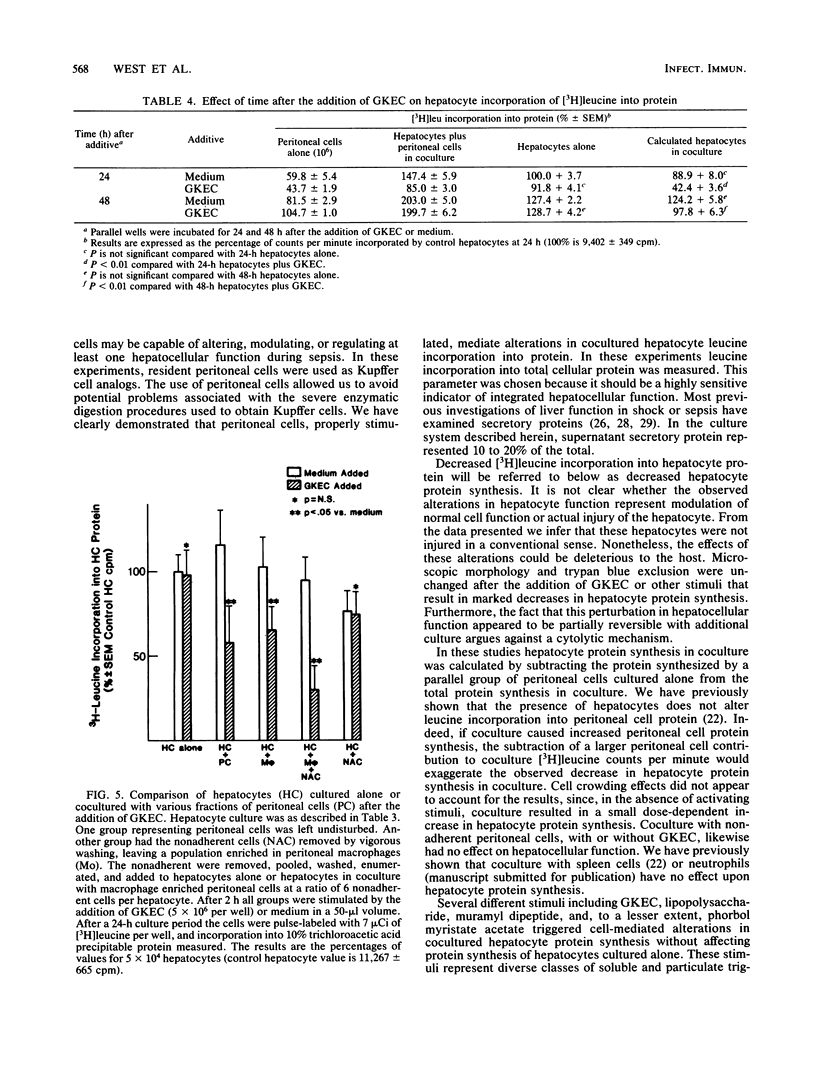
Hepatic dysfunction is a poorly understood and highly lethal component of multiple-system organ failure. Both in vivo and in vitro studies of "liver" function have generally neglected hepatocyte-Kupffer cell interactions. In the following experiments, isolated hepatocytes were cocultivated with unstimulated peritoneal cells, predominately macrophages, which served as a readily available Kupffer cell analog. Coculture of hepatocytes with peritoneal cells resulted in little or no change in [3H]leucine incorporation into hepatocyte protein. When gentamicin-killed Escherichia coli cells (GKEC) were added to coculture, there was a marked decrease in hepatocyte [3H]leucine incorporation. In contrast, GKEC added to hepatocytes alone had no effect. Kinetic data revealed an 8-h delay before any significant decrease in leucine incorporation into hepatocyte protein after the addition of GKEC to the coculture. The maximal decrease in hepatocyte [3H]leucine incorporation occurred 24 h after GKEC were added. The decrease observed 24 h after GKEC were added disappeared almost completely after 48 h of coculture. Similar alterations in cocultured hepatocyte protein synthesis were observed after the addition of phorbol myristate acetate, lipopolysaccharide, or muramyl dipeptide, a component of bacterial peptidoglycan. Hepatocyte viability by trypan blue exclusion was unchanged, and gross morphology by light or electron microscopy was unaffected. We propose that during sepsis, macrophages (Kupffer cells) respond to circulating microbial products and mediate alterations in hepatocyte function. These experiments underscore the important role of Kupffer cell function in attempts to understand hepatic malfunction in multiple-system organ failure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Bernheim H. A., Block L. H., Atkins E. Fever: pathogenesis, pathophysiology, and purpose. Ann Intern Med. 1979 Aug;91(2):261–270. doi: 10.7326/0003-4819-91-2-261. [DOI] [PubMed] [Google Scholar]
- Bouwens L., Baekeland M., Wisse E. Importance of local proliferation in the expanding Kupffer cell population of rat liver after zymosan stimulation and partial hepatectomy. Hepatology. 1984 Mar-Apr;4(2):213–219. doi: 10.1002/hep.1840040208. [DOI] [PubMed] [Google Scholar]
- Bucana C. D., Hoyer L. C., Schroit A. J., Kleinerman E., Fidler I. J. Ultrastructural studies of the interaction between liposome-activated human blood monocytes and allogeneic tumor cells in vitro. Am J Pathol. 1983 Jul;112(1):101–111. [PMC free article] [PubMed] [Google Scholar]
- Cerra F. B., Siegel J. H., Border J. R., Wiles J., McMenamy R. R. The hepatic failure of sepsis: cellular versus substrate. Surgery. 1979 Sep;86(3):409–422. [PubMed] [Google Scholar]
- Clowes G. H., Jr, George B. C., Villee C. A., Jr, Saravis C. A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N Engl J Med. 1983 Mar 10;308(10):545–552. doi: 10.1056/NEJM198303103081001. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Ritchie D. G. A regulatory pathway for fibrinogen biosynthesis involving an indirect feedback loop. Ann N Y Acad Sci. 1982;389:308–322. doi: 10.1111/j.1749-6632.1982.tb22146.x. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Sparkes R. S., Golde D. W. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science. 1978 Sep 8;201(4359):937–938. doi: 10.1126/science.356266. [DOI] [PubMed] [Google Scholar]
- Horiuti Y., Nakamura T., Ichihara A. Role of serum in maintenance of functional hepatocytes in primary culture. J Biochem. 1982 Dec;92(6):1985–1994. doi: 10.1093/oxfordjournals.jbchem.a134130. [DOI] [PubMed] [Google Scholar]
- Ichihara A., Nakamura T., Tanaka K., Tomita Y., Aoyama K., Kato S., Shinno H. Biochemical functions of adult rat hepatocytes in primary culture. Ann N Y Acad Sci. 1980;349:77–84. doi: 10.1111/j.1749-6632.1980.tb29517.x. [DOI] [PubMed] [Google Scholar]
- Jaffe C. J., Vierling J. M., Jones E. A., Lawley T. J., Frank M. M. Receptor specific clearance by the reticuloendothelial system in chronic liver diseases. Demonstration of defective C3b-specific clearance in primary biliary cirrhosis. J Clin Invest. 1978 Nov;62(5):1069–1077. doi: 10.1172/JCI109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., West M. A., Harty J. T., Cerra F. B., Simmons R. L. Modulation of hepatocyte protein synthesis during co-cultivation with macrophage-rich peritoneal cells in vitro. Arch Surg. 1985 Feb;120(2):180–186. doi: 10.1001/archsurg.1985.01390260044007. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. II. Dexamethasone enhanced longevity and maintenance of morphology. In Vitro. 1976 Dec;12(12):821–832. doi: 10.1007/BF02796367. [DOI] [PubMed] [Google Scholar]
- Lebreton J. P., Joisel F., Raoult J. P., Lannuzel B., Rogez J. P., Humbert G. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979 Oct;64(4):1118–1129. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Schreiber R. D., La Forest A. C., Ulevitch R. J. Suppression of ACTH-induced steroidogenesis by supernatants from LPS-treated peritoneal exudate macrophages. J Immunol. 1983 Jun;130(6):2757–2762. [PubMed] [Google Scholar]
- McAdam K. P., Li J., Knowles J., Foss N. T., Dinarello C. A., Rosenwasser L. J., Selinger M. J., Kaplan M. M., Goodman R., Herbert P. N. The biology of SAA: identification of the inducer, in vitro synthesis, and heterogeneity demonstrated with monoclonal antibodies. Ann N Y Acad Sci. 1982;389:126–136. doi: 10.1111/j.1749-6632.1982.tb22131.x. [DOI] [PubMed] [Google Scholar]
- McMenamy R. H., Birkhahn R., Oswald G., Reed R., Rumph C., Vaidyanath N., Yu L., Cerra F. B., Sorkness R., Border J. R. Multiple systems organ failure: I. The basal state. J Trauma. 1981 Feb;21(2):99–114. doi: 10.1097/00005373-198102000-00003. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Keeton D. G., Webber B. L., Pathol F. F., Saunders S. J. Jaundice in severe bacterial infection. Gastroenterology. 1976 Jul;71(1):94–97. [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner R. G., Tanner A. R., Keyhani A. H., Wright R. A comparative study of lysosomal enzyme activity in monocytes and Kupffer cells isolated simultaneously in a rat model of liver injury. Clin Exp Immunol. 1981 Feb;43(2):376–380. [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. G., Levy B. A., Adams M. A., Fuller G. M. Regulation of fibrinogen synthesis by plasmin-derived fragments of fibrinogen and fibrin: an indirect feedback pathway. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1530–1534. doi: 10.1073/pnas.79.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Role of the Kupffer cells in local and systemic immune responses. Gastroenterology. 1981 Apr;80(4):854–860. [PubMed] [Google Scholar]
- Rupp R. G., Fuller G. M. The effects of leucocytic and serum factors on fibrinogen biosynthesis in cultured hepatocytes. Exp Cell Res. 1979 Jan;118(1):23–30. doi: 10.1016/0014-4827(79)90579-2. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Souhami R. L., Bradfield J. W. The recovery of hepatic phagocytosis after blockade of Kupffer cells. J Reticuloendothel Soc. 1974 Aug;16(2):75–86. [PubMed] [Google Scholar]
- Soyka L. F., Hunt W. G., Knight S. E., Foster R. S., Jr Decreased liver and lung drug-metabolizing activity in mice treated with Corynebacterium parvum. Cancer Res. 1976 Dec;36(12):4425–4428. [PubMed] [Google Scholar]
- Tanaka K., Sato M., Tomita Y., Ichihara A. Biochemical studies on liver functions in primary cultured hepatocytes of adult rats. I. Hormonal effects on cell viability and protein synthesis. J Biochem. 1978 Oct;84(4):937–946. doi: 10.1093/oxfordjournals.jbchem.a132207. [DOI] [PubMed] [Google Scholar]
- Vermillion S. E., Gregg J. A., Baggenstoss A. H., Bartholomew L. G. Jaundice associated with bacteremia. Arch Intern Med. 1969 Nov;124(5):611–618. [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Pekarek R. S., Thompson W. L., Curnow R. T., Beall F. A., Zenser T. V., DeRubertis F. R., Beisel W. R. A protein from polymorphonuclear leukocytes (LEM) which affects the rate of hepatic amino acid transport and synthesis of acute-phase globulins. Endocrinology. 1975 Mar;96(3):651–661. doi: 10.1210/endo-96-3-651. [DOI] [PubMed] [Google Scholar]


