Abstract
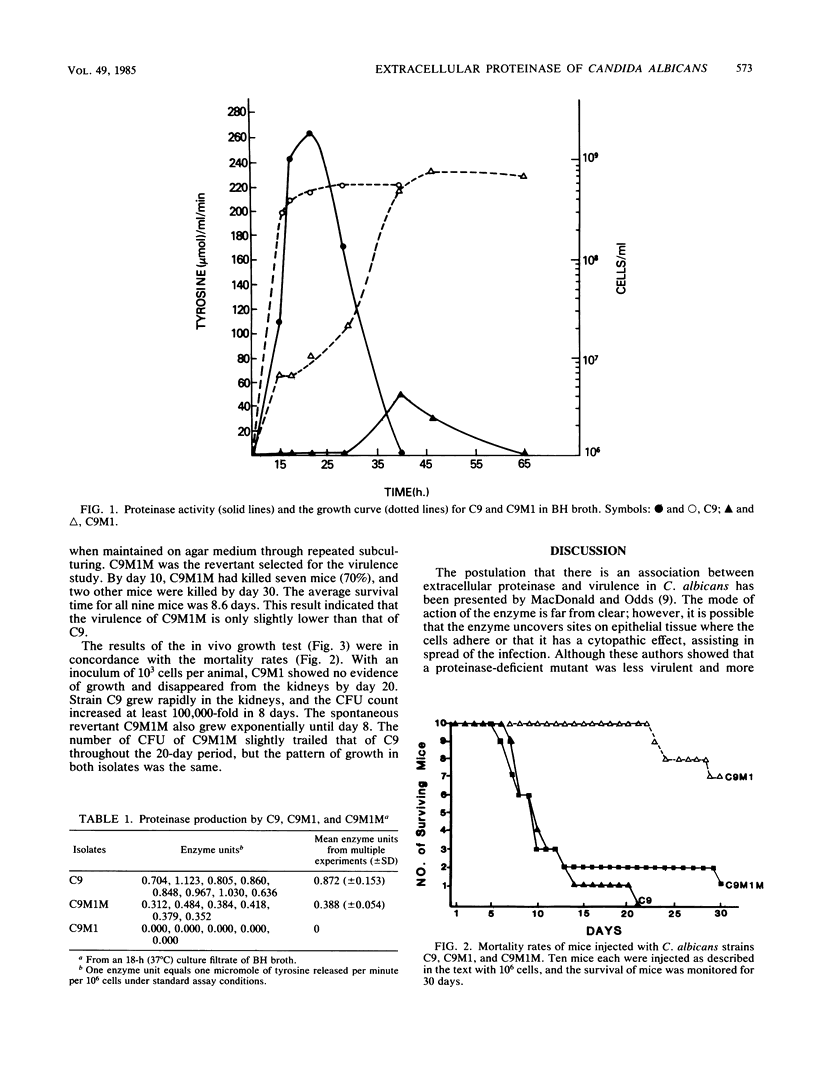
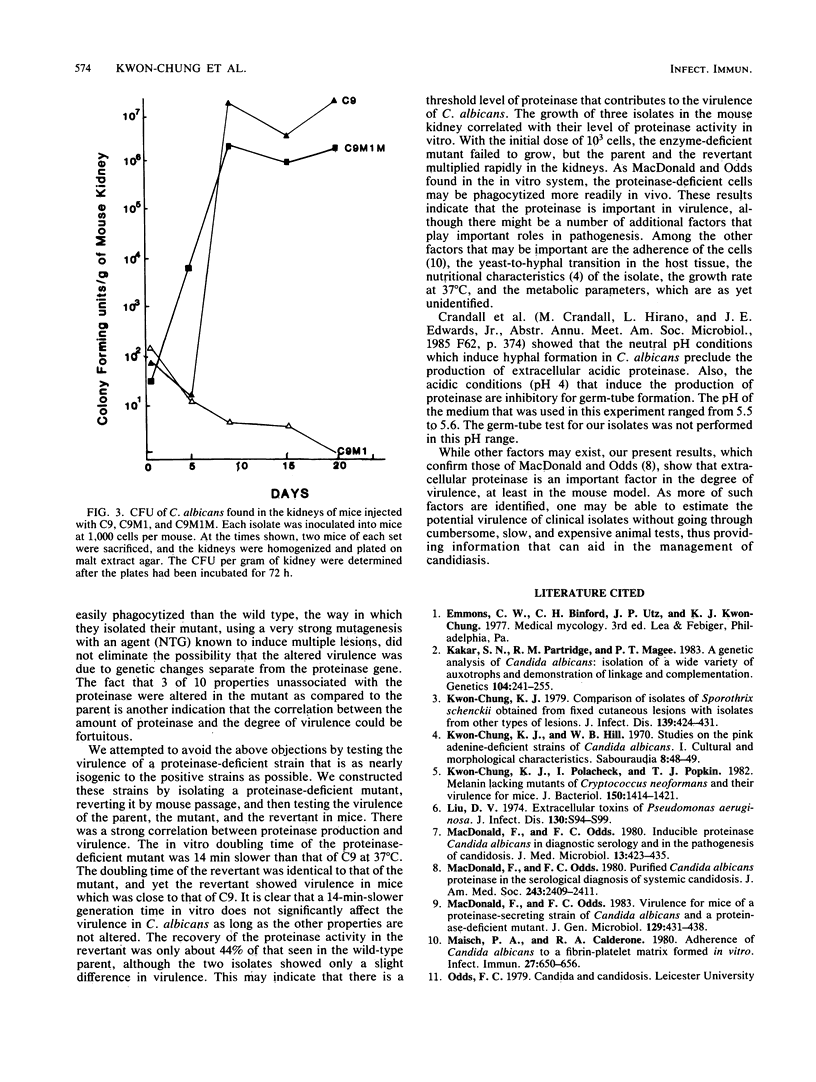
The relationship between extracellular proteinase and the virulence for mice in Candida albicans was studied by using a set of three isolates. The set included a proteinase-producing parent (C9), a proteinase-deficient mutant derived from C9 by nitrous acid treatment (C9M1), and a spontaneous revertant (C9M1M) obtained by mouse passage of C9M1. The morphological markers and the carbon assimilation pattern were identical in these isolates. Isolate C9 produced a high level of proteinase in vitro and caused fatal infection (100%) within 21 days. The mutant produced no detectable enzymes in vitro, and all mice survived until day 22. Only 30% of the mice infected with C9M1 died between day 23 and 30. The isolates recovered from the dead mice were found to be proteinase sufficient, indicating that the mice died after the organism in tissue had reverted. The C9M1M isolate produced proteinase in vitro at 44% the level of C9 and induced fatal infection in 90% of the mice within 30 days. The number of CFU recovered from the kidneys correlated with the level of proteinase produced in vitro and, in turn, the rate of fatal infection produced by the isolates. These results support a previous observation indicating that proteinase activity is one of the virulence factors associated with C. albicans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kakar S. N., Partridge R. M., Magee P. T. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983 Jun;104(2):241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J Infect Dis. 1979 Apr;139(4):424–431. doi: 10.1093/infdis/139.4.424. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Purified Candida albicans proteinase in the serological diagnosis of systemic candidosis. JAMA. 1980 Jun 20;243(23):2409–2411. [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Tegeler R., Trost M. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia. 1982 Sep;20(3):233–244. doi: 10.1080/00362178285380341. [DOI] [PubMed] [Google Scholar]
- Staib F. Serum-Protein-Agar-pH 4,2 und 5,0 für Sprosspilze. Zentralbl Bakteriol Orig. 1964 Dec;195(2):265–267. [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]


