Abstract
Background
Previous research has demonstrated that the cerebellum is involved in emotive and cognitive processes. Furthermore, recent findings suggest high-frequency repetitive transcranial magnetic stimulation (rTMS) to the cerebellum has mood-improving properties. We sought to further explore the effects of cerebellar high-frequency rTMS on implicit processing of emotional stimuli and mood.
Methods
In a double-blind, crossover study, 15 healthy volunteers received 15 minutes of 20 Hz (5 s on, 5 s off) rTMS over the medial cerebellum, occipital cortex or sham in a randomized counterbalanced order on 3 consecutive days. A masked emotional faces response task measured implicit emotional processing of happy, fearful and neutral facial expressions. We used positive and negative affect scales to evaluate rTMS-related changes in mood.
Results
High-frequency rTMS over the cerebellum was associated with significant increases in masked emotional responses to happy facial expressions only. We observed no changes in consciously experienced mood.
Limitations
Although the sham rTMS served as our baseline measurement, additional pre-rTMS data showing that reaction time increases immediately after cerebellar rTMS would have made our results more compelling.
Conclusion
The results replicate and extend previous findings by establishing a direct relation between the cerebellum and emotive information-processing. The parallel between the present effects of high-frequency cerebellar rTMS and short-term antidepressant therapy regarding the change in implicit processing of positive stimuli in the absence of mood changes is notable and warrants further research.
Abstract
Contexte
Des recherches antérieures ont démontré que le cervelet joue un rôle dans les processus émotionnels et cognitifs. De récentes découvertes permettent également de penser que la stimulation magnétique transcrânienne répétitive (SMTr) de haute fréquence appliquée au cervelet exercerait des effets positifs sur l'humeur. Nous avons voulu explorer plus en profondeur les effets de la SMTr sur le cervelet, sur le traitement implicite des stimuli émotionnels et sur l'humeur.
Méthodes
Dans le cadre d'une étude à double insu avec permutation des groupes, 15 volontaires en bonne santé ont reçu 15 minutes de SMTr à 20 Hz (stimulation 5 s, arrêt 5 s) au niveau de la partie médiane du cervelet ou du cortex occipital, ou une stimulation simulée, en séquences randomisées contrebalancées, 3 jours consécutifs. Un test masqué de décodage de l'émotion exprimée par des visages a permis de mesurer le traitement émotionnel implicite des expressions faciales heureuses, craintives et neutres. Nous avons utilisé des échelles d'évaluation des effets positifs et négatifs pour mesurer les changements de l'humeur liés à la SMTr.
Résultats
La SMTr de haute fréquence appliquée au cervelet a été associée à des augmentations significatives des réponses émotionelles masquées, mais uniquement des réponses aux expressions faciales heureuses. Nous n'avons observé aucun changement de l'humeur consciente.
Limites
Même si la SMTr simulée nous a servi de mesure de départ, d'autres données pré-SMTr montrant que le temps de réaction augmente immédiatement après l'application de la SMTr au cervelet auraient donné plus de poids à nos résultats.
Conclusion
Les résultats reproduisent et élargissent les conclusions antérieures en établissant un lien direct entre le cervelet et le traitement émotionnel de l'information. Le parallèle entre les effets actuels de l'application de la SMTr de haute fréquence au cervelet et le traitement antidépresseur à court terme, en ce qui a trait à une modification du traitement implicite des stimuli positifs en l'absence de labilité de l'humeur, est notable et justifie que l'on approfondisse la recherche.
Introduction
The concept that the cerebellum is implicated in the experience and regulation of emotions and mood was posited more than half a century ago.1,2 The first empirical report of cerebellar involvement in the experience of emotions included a study of a patient who underwent electrical stimulation of the dentate nucleus and superior peduncle and experienced negative feelings.3 The link between the cerebellum and emotions was further strengthened by the seminal work of Heath and colleagues,4–7 demonstrating that chronic electrical stimulation of the superficial parts of vermis normalized behaviour in severely emotionally disturbed patients. A neuroanatomical basis for cerebellar involvement in emotive processes is grounded in the multiple direct and indirect connections of the cerebellum to limbic and cortical areas of the brain. Functional neuroimaging studies have also demonstrated consistent cerebellar activation during emotive processing and mood.8 One of the prevailing models proposes that the cerebellum functions as a “general-purpose modulator” in governing mental activities (for a review see Andreasen and colleagues9). Further evidence of a direct link between the human cerebellum and emotive processes has been provided by repetitive transcranial magnetic stimulation (rTMS) studies. In a previous study, it was shown that a single session of high-frequency rTMS over the cerebellum had positive effects on mood in healthy volunteers.10 Interestingly, mood elevations often go accompanied by increased attention for implicit positive stimuli that can even occur before actual changes in mood.11 These changes in implicit attention for positive stimuli can, for instance, be evaluated by measuring reaction times to the colour naming of a mask that is preceded by a short (nonconscious) presentation of a happy facial expression.12 Increased emotional responsiveness for implicit positive stimuli would then result in longer reaction times to the colour naming of the mask preceded by a happy facial expression and allow researchers to gather insights into information processes associated with mood.
Building on the previous high-frequency rTMS study, we recently showed that low-frequency rTMS to the medial part of the cerebellum in healthy volunteers impairs emotion regulation and augments negative mood.13 These findings are not only in accordance with the alleged opposite actions of high-and low-frequency stimulation parameters, but also demonstrate the modulatory effects of a single session of cerebellar rTMS on behaviour.
We conducted a sham and occipital cortex controlled, double-blind, crossover study to further explore the effects of high-frequency rTMS over the cerebellum on emotive information processing and mood. In keeping with our previous findings, we hypothesized that a single session of high frequency cerebellar rTMS would increase self-reported positive mood and facilitate information-processing of positive emotional stimuli.
Methods
Participants
We enrolled healthy, nonsmoking young women in the study. We obtained written informed consent and paid the volunteers for their participation. The study was approved by the medical ethical committee of the Utrecht University in accordance with the declaration of Helsinki. All volunteers were unaware of the hypotheses being tested in the study.
Transcranial magnetic stimulation
We performed high-frequency rTMS using a biphasic magnetic brain stimulator (maximum output 2300 A peak / 1750 VAC peak) with an iron core coil (Neotonus). Maximum magnetic field strength was 2 Tesla. We performed sham rTMS using an identical coil with a built-in aluminum plate directly underneath the iron core (Neotonus). The coil mimics the sound click and sensation of real TMS, but the brain is shielded from actual stimulation.
The masked emotional faces response task
The masked emotional faces response task requires participants to name the colour of the ink in which the mask is printed. Performance in terms of slowing down or speeding up colour naming varies as a function of the participant's motivational state and the rapid emotional face presentation (14 ms) prior to the mask.12 In this task, the motivational state of the individual is presumed to direct pre-attentive (automatic) reactions to the emotional facial expressions and influence the colour naming of the subsequent presentation of the mask.14,15 Thus, slower colour naming of the masking stimulus following happy facial expressions is indicative of increased appetitive motivation and reward sensitivity, whereas the opposite holds for relatively faster colour naming.16 Figure 1 depicts the outline of the masked emotional faces response task.
Fig. 1: The masked emotional faces response task.
The general idea of the task is that presenting emotional facial expressions outside conscious awareness prevents cognitive elaborative strategies and subsequently gains access into the core aspects of the individual's motivational stance.
We used stimuli from Ekman and Friesen's Pictures of facial affect17 and Lundqvist and colleagues' Karolinska directed emotional faces set.18 In the masked emotional faces response task, we displayed a fixation point for 750 ms, followed by a 14-ms presentation of a happy, fearful or neutral facial expression that we then replaced by a masking stimulus. Masking stimuli included randomly cut, reassembled and rephotographed pictures of faces.
We presented 30 happy, 30 fearful and 30 neutral faces in random order, and the inter-trial interval randomly varied between 1500 and 2500 ms. All stimuli (14 × 9 cm) were coloured either red, green or blue and projected in the centre of a 17-inch computer screen (70 Hz refresh rate) on a black background at a distance of 150 cm. We instructed participants to name the colour of the masking stimulus as fast as possible. For optimizing stress-related workload, we used a rapid externally paced version of the task with a termination of mask display after 300 ms.19 We used the reaction time as the dependent variable.
To check for the absence of conscious awareness, we used a 3-alternative, forced-choice happy–fear–neutral recognition check (3AFC). We showed a random set of 60 masked facial expressions to each participant. Prior to the check, we explicitly told participants that the set contained 20 happy, 20, fearful and 20 neutral faces, and we instructed them to indicate whether the presented stimulus was a happy, fearful or neutral emotional expression by pushing 1, 2 or 3, respectively, on the keypad. We set the critical level of perceptual threshold at 26 correct trials, as indicated by a nonparametric binomial test (n = 60, test proportion = 0.33).
Self-reported mood questionnaire
We assessed consciously experienced mood using the positive and negative affect schedule.20 The questionnaire consisted of 20 items and visual analogue scales (0 = not at all, 100 = extremely).
Procedure
Participants received 15 minutes of 20 Hz rTMS (5 s on, 5 s off, 9000 pulses per session) over the midline cerebellum. Sham rTMS over the midline cerebellum and real rTMS over the occipital cortex served as the inactive and active control conditions, respectively. The current in the coil was directed downward to maximize cerebellar stimulation.21
We defined the midline cerebellum target site as the point located 1 cm below the inion.22,23 The occipital cortex target site was located 3 cm above the inion. Stimulation intensity was 80% of the individual motor threshold. We randomized the order of sessions and counterbalanced it among participants. Sessions were 24 hours apart and controlled for daytime. Volunteers underwent 1 condition per session. Stimulation parameters were in accordance with the safety guidelines formulated by the International Federation of Clinical Neurophysiology (www.ifcn.info).
On a separate day prior to the testing sessions, we used a safety-screening list to check for contraindications, and we assessed the health of all participants using a standard interview.24 In addition, we explained safety issues and the experimental procedures to each participant and obtained informed consent. We assessed handedness using the Edinburgh handedness inventory,25 and we determined the average motor threshold of the left and right hemisphere using the 5-step motor threshold estimation procedure described by Schutter and van Honk.26 On testing days, we instructed participants to refrain from taking psychotropic substances, including alcohol, coffee, tea and chocolate, for 2 hours before the experiment. We administered the positive and negative affect schedule questionnaire immediately after high-frequency rTMS. Next, participants performed the masked emotional faces response task. Finally, at the end of the final session participants performed the 3AFC, and we debriefed them and paid them for participation.
Statistical analysis
We used a general linear model for repeated-measurements with 3 levels using cerebellar, occipital and sham rTMS as within-subject factors and order of rTMS conditions as the between-subject factor. We applied Greenhouse–Geisser correction (degrees of freedom > 1) to the p values and reported the ε. We performed post-hoc significance testing with simple paired-sample t tests. We set the α level of significance at ≤ 0.05, 2-tailed.
Results
Participants
All 15 participants were aged 18–22 (mean 20.4, standard deviation [SD] 1.9) years, right-handed (mean 45.9, SD 2.7, as per Edinburgh handedness inventory) and used oral contraceptives. The mean motor threshold of the left and right hemisphere was 57.7 (SD 10.0). None had a history of psychiatric or neurologic conditions, and all had normal or corrected-to-normal vision.
Procedure
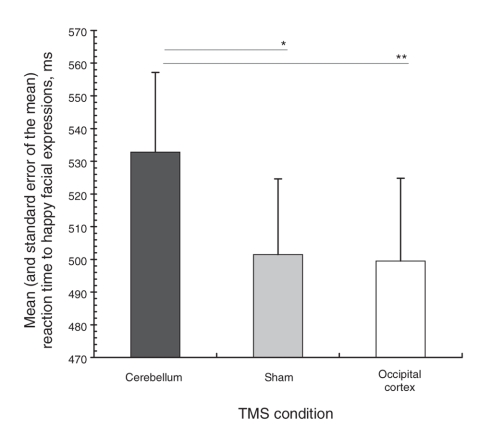
TMS was well tolerated and, although some participants noticed differences in sensation between the sessions, blinding was effective because participants were not able to separate the experimental from the control conditions when interviewed. None of the volunteers passed the masked emotional faces awareness check criterion, and we enetered data on all 15 participants in the statistical analyses. We observed a significant main effect of rTMS on reaction times to masked happy facial expressions (F2,18 = 4.23, p = 0.032, ε = 0.99); however, we observed no significant effect of the order of conditions (F10,10 = 1.16, p = 0.37, ε = 0.99). Post-hoc paired-samples t tests revealed that reaction times were significantly increased after cerebellar compared with sham (t14 = 2.5, p = 0.021) and occipital cortex rTMS (t14 = 2.3, p = 0.037). Reaction times between the sham and occipital rTMS condition did not statistically differ (t14 = 0.18, p = 0.86). We observed no main effects of rTMS on reaction times for the masked neutral (F2,18 = 1.36, p = 0.28, ε = 0.85) and fearful facial expressions (F2,18 = 1.67: p = 0.22, ε = 0.69). We found no order effects of TMS in the masked neutral and fearful facial expressions (p > 0.36). Figure 2 shows the mean and standard error of the mean colour naming reaction times to masked facial expressions for the rTMS conditions.
Fig. 2: Mean and standard error of the mean reaction times showing increased emotional responses to masked happy facial expressions after 15 minutes of high-frequency repetitive transcranial magnetic stimulation (rTMS) over the cerebellum. *p = 0.021, **p = 0.037.
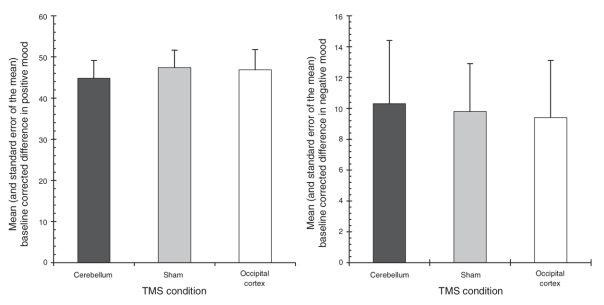
Our repeated-measures multivariate analysis of variance did not yield significant differences between the rTMS conditions on positive (F2,18 = 0.31, p = 0.68, ε = 0.75) and negative mood scores (F2,18 = 0.10, p = 0.90, ε = 0.98), as seen in Figure 3. We observed no significant order effects of TMS in the mood scores (both p values > 0.38).
Fig. 3: Mean and standard error of the mean baseline corrected scores of consciously experienced positive (left panel) and negative mood (right panel) across the transcranial magnetic stimulation (TMS) conditions.
Discussion
Our goal was to examine the effects of high-frequency rTMS over the cerebellum on responses to masked emotional facial expressions and consciously experienced mood. Results showed that cerebellar rTMS, compared with occipital cortex and sham rTMS, resulted in the enhanced implicit processing of happy facial expressions without changes in self-reported mood. Our findings concur with those of pharmacological studies that found selective increases in implicit processing of positive emotional material, but no changes in consciously experienced mood related to antidepressant therapy in healthy nondepressed volunteers.11,27,28 Furthermore, our results are in agreement with findings of increased attentional biases (i.e., slower reaction times) to happy facial expressions in nondepressed versus depressed individuals.29 In contrast, the absence of a significant increase in self-reported positive mood is not consistent with our previous work in which we did find a positive effect on mood. Notably, the fact that the current rTMS findings were confined to the implicit level of information-processing coincides with the general idea that the early effects of antidepressants are manifested at the level of implicit and automatic aspects of information-processing rather than the conscious experience of mood.28
Our results further support the involvement of the cerebellum in affective information-processing. Admittedly, our study design does not permit us to draw strong inferences on the underlying biological mechanisms. There are at least 3 lines of indirect evidence suggesting that the effects of high-frequency rTMS to the medial cerebellum involve the brain's reward circuitry. First, the vermis part of the human cerebellum has projections to the ventral tegmental area of the midbrain, showing that the vermis can modulate mesolimbic areas via dopaminergic fibre bundles.30 Second, a comparative study has demonstrated enhanced dopamine turnover in the nucleus accumbens to electric stimulation of the cerebellar vermis in rats.31,32 The finding is further supported by observations of increased dopamine metabolite homovanillic acid in the cerebrospinal fluid of 2 patients with movement disorders who received chronic electrical cerebellar stimulation.33 Third, high-frequency electrical stimulation of Purkinje cells in the cerebellar cortex selectively modulates dopaminergic activity in the prefrontal cortex.34
Together, these findings suggest that the cerebellum plays a significant role in the modulation of brain regions associated with cognition and emotion.35,36 Finally, it is proposed that the cerebellum may be a suitable entry point for targeting dopaminergic pathways in the mesolimbic and mesocortical systems as an alternative way to treat depression, for instance, with high-frequency rTMS.37,38
Limitations
Our study has some limitations. First, we used a Likert-type scale to assess consciously experienced mood. Previous research has demonstrated that slight but systematic changes in mood may remain unnoticed when using Likert-type scales.39,40 Second, the fewer number of pulses that we applied in the present study (i.e., 9000 v. 20 000 pulses in our earlier study) to prevent coil heating and minimize participants' discomfort may explain the absence of a significant increase in self-reported positive mood among participants in the present study. Analogous to the therapeutic onset delays often observed in drug as well as in rTMS treatment studies, a greater number of magnetic pulses at higher intensities might be necessary to obtain changes in consciously experienced mood. Third, although the sham rTMS served as our baseline measurement, additional pre-rTMS data showing that reaction time increases immediately after cerebellar rTMS would have made the results more compelling. Finally, it should be noted that the conclusion of increased emotional responses is indirectly drawn from increased reaction times. Except for the fact that our results suggest a role for the cerebellum in the implicit processing of positive emotional stimuli, the brain mechanisms responsible for the change in emotional responsiveness remain speculative and additional research on this matter is needed.41,42
In sum, the results of our high-frequency rTMS study replicate and extend previous findings by establishing a direct relation between the cerebellum and emotive information processing. The parallel between the present effects of high-frequency cerebellar rTMS and short-term antidepressant therapy regarding the change in implicit processing of positive stimuli in the absence of mood changes is notable and warrants further research.
Acknowledgments
This work was supported by an Innovational Research Grant (VENI 451-04-070) from the Netherlands Organization for Scientific Research.
Footnotes
Contributors: Dr. Schutter designed the study. Dr. Schutter and Mr. Hoppenbrouwers analyzed the data and wrote the article. All authors acquired the data, reviewed the article and gave final approval for publication.
Competing interests: None declared.
Correspondence to: Dr. D.J.L.G. Schutter, Experimental Psychology, Helmholtz Institute, Utrecht University, Heidelberglaan 2, 3584CS Utrecht, The Netherlands; fax 31 30 253 4511; d.schutter@uu.nl
References
- 1.Snider RS. A tectocerebellar pathway. Anat Rec 1945;91:299.
- 2.Anand BK, Malhorta CL, Singh B, et al. Cerebellar projections to the limbic system. J Neurophysiol 1959;22:451-8. [DOI] [PubMed]
- 3.Nashold BS, Slaughter DG. Effects of stimulating or destroying the deep cerebellar regions in man. J Neurosurg 1969;31:172-86. [DOI] [PubMed]
- 4.Heath RG. Modulation of emotion with a brain pacemaker: treatment for intractable psychiatric illness. J Nerv Ment Dis 1977;165:300-17. [PubMed]
- 5.Heath RG, Dempsey CW, Fontana CJ, et al. Cerebellar stimulation: effects on septal region, hippocampus and amygdala of cats and rats. Biol Psychiatry 1978;13:501-29. [PubMed]
- 6.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res 1976;2:133-46. [DOI] [PubMed]
- 7.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 2001;21:700-12. [DOI] [PMC free article] [PubMed]
- 8.Habel U, Klein M, Kellermann T, et al. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage 2005;26:206-14. [DOI] [PubMed]
- 9.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81-8. [DOI] [PMC free article] [PubMed]
- 10.Schutter DJLG, van Honk J, d'Alfonso AAL, et al. High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett 2003;336:73-6. [DOI] [PubMed]
- 11.Harmer CJ, Hill SA, Taylor MJ, et al. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry 2003;160:990-2. [DOI] [PubMed]
- 12.van Honk J, Tuiten A, de Haan EHF. Attentional biases for angry faces: relationships to trait anger and anxiety. Cogn Emot 2001;15: 279-97.
- 13.Schutter DJLG, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. Cerebellum 2008 Oct 15 [Epub ahead of print]. [DOI] [PubMed]
- 14.Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol 1994;45:25-50. [DOI] [PubMed]
- 15.van Honk J, de Haan EHF. Cortical and subcortical routes for conscious and unconscious processing of emotional faces. In: De Gelder B, Heywood CA, de Haan E, editors. Varieties of unconscious processing. Oxford: Oxford University Press; 2000. p. 222-37.
- 16.Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull 2005;31:121-35. [DOI] [PubMed]
- 17.Ekman P, Friesen W. Pictures of facial effect. Palo Alto (CA): Consulting Psychologist Press; 1976.
- 18.Lundqvist A, Flykt A, Öhman A. The Karolinska directed emotional faces. Stockholm: Karolinska Hospital; 1998.
- 19.Renaud P, Blondin JP. The stress of Stroop performance: physiological and emotional responses to color-word interference, task pacing, and pacing speed. Int J Psychophysiol 1997;27:87-97. [DOI] [PubMed]
- 20.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063-70. [DOI] [PubMed]
- 21.Ugawa Y, Uesaka Y, Terao Y, et al. Magnetic stimulation over the cerebellum in humans. Ann Neurol 1995;37:703-13. [DOI] [PubMed]
- 22.Schutter DJLG, Kammers MPM, Enter D, et al. A case of illusory own-body perception after transcranial magnetic stimulation over the cerebellum. Cerebellum 2006;5:238-40. [DOI] [PubMed]
- 23.Torriero S, Oliveri M, Koch G, et al. Interference of left and right cerebellar rTMS with procedural learning. J Cogn Neurosci 2004;16:1605-11. [DOI] [PubMed]
- 24.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 2001;112:720. [DOI] [PubMed]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97-113. [DOI] [PubMed]
- 26.Schutter DJLG, van Honk J. A standardized motor threshold estimation procedure for transcranial magnetic stimulation. J ECT 2006;22:176-8. [DOI] [PubMed]
- 27.Harmer CJ, Shelley NC, Cowen PJ, et al. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry 2004;161:1256-63. [DOI] [PubMed]
- 28.Norbury R, Mackay CE, Cowen PJ, et al. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br J Psychiatry 2007;190:531-2. [DOI] [PubMed]
- 29.Gotlib IH, McLachlan AL, Katz AN. Biases in visual attention in depressed and non-depressed individuals. Cogn Emot 1988;2:185-200.
- 30.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res 1976;2:133-46. [DOI] [PubMed]
- 31.Albert TJ, Dempesy CW, Sorenson CA. Anterior cerebellar vermal stimulation: effect on behavior and basal forebrain neurochemistry in rat. Biol Psychiatry 1985;20:1267-76. [DOI] [PubMed]
- 32.Dempsey CW, Richardson DE. Paleocerebellar stimulation induces in vivo release of endogenously synthesized [3H]dopamine and [3H]norepinephrine from rat caudal dorsomedial nucleus accumbens. Neuroscience 1987;21:565-71. [DOI] [PubMed]
- 33.Tabaddor K, Wolfson L, Sharpless N, et al. Ventricular fluid homovanillic acid and 5-hydroxyindoleacetic acid concentrations in patients with movement disorders. Neurology 1978;28:1249-53. [DOI] [PubMed]
- 34.Mittleman G, Goldowitz D, Heck DH, et al. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse 2008;62:544-50. [DOI] [PMC free article] [PubMed]
- 35.Schmahmann JD. The role of the cerebellum on affect and psychosis. J Neurolinguistics 2000;13:189-214.
- 36.Schutter DJLG, van Honk J. The cerebellum on the rise in human emotion. Cerebellum 2005;4:290-4. [DOI] [PubMed]
- 37.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007;64:327-37. [DOI] [PubMed]
- 38.Schutter DJLG, van Honk J. A framework for targeting alternative brain regions with repetitive transcranial magnetic stimulation in the treatment of depression. J Psychiatry Neurosci 2005;30:91-7. [PMC free article] [PubMed]
- 39.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol 1974;47:211-18.
- 40.Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology 1971;10:181-91. [DOI] [PubMed]
- 41.Harmer CJ, Mackay CE, Reid CB, et al. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 2006;59:816-20. [DOI] [PubMed]
- 42.Schutter DJLG, van Honk J. Increased positive emotional memory after repetitive transcranial magnetic stimulation over the orbitofrontal cortex. J Psychiatry Neurosci 2006;31:101-4. [PMC free article] [PubMed]