Abstract
Activation of the inflammatory immune system provokes numerous neuroendocrine and neurotransmitter changes, many of which are similar to those provoked by physical or psychological stressors. These findings, among others, have led to the suggestion that the brain translates immune activation much as if it were a stressor. In this review, I provide synopses of the effects of traditional stressors on the release of corticotropin-releasing hormones at hypothalamic and extrahypothalamic sites, variations of serotonin and its receptors and changes of brain-derived neurotrophic factor (BDNF). These effects are similar to those elicited by activation of the inflammatory immune system, particularly the impact of the immune-signalling molecules interleukin-1β, interleukin-6, tumour necrosis factor-α and interferon-α on neuroendocrine, neurotransmitter and BDNF function. In addition, it is reported that stressors and cytokines may synergistically influence biological and behavioural processes and that these treatments may have long-term ramifications through the sensitization of processes associated with stress responses. Finally, I present an overview of the depressogenic actions of these cytokines in rodent models and in humans, and I provide provisional suggestions (and caveats) about the mechanisms by which cytokines and stressors might culminate in major depressive disorder.
Abstract
L'activation du système immunitaire et de la réponse inflammatoire provoque de nombreux phénomènes affectant le système neuroendocrinien et les neurotransmetteurs, dont bon nombre se comparent aux effets des stresseurs physiques ou psychologiques. Ces observations ont entre autres mené à l'hypothèse selon laquelle le cerveau interprète l'activation immunitaire comme un stresseur. Dans la présente synthèse, je propose un résumé des effets des stresseurs traditionnels sur la sécrétion de corticolibérine aux sièges hypothalamiques et extrahypothalamiques, sur la fluctuation des taux de sérotonine et de ses récepteurs et sur les variations du facteur neurotrophique d'origine cérébrale (BDNF, brain-derived neurotrophic factor). Ces effets sont similaires à ceux qu'entraîne l'activation du système immunitaire et de la réponse inflammatoire, notamment l'impact des molécules signal immunitaires interleukine-1β, interleukine-6, facteur de nécrose tumorale alpha et interféron alpha sur la fonction neuroendocrinienne, les neurotransmetteurs et le BDNF. On rapporte de plus que les stresseurs et les cytokines peuvent exercer une influence synergique sur les processus biologiques et comportementaux et que ces phénomènes pourraient avoir des répercussions à long terme par le biais d'une sensibilisation des processus associés aux réponses au stress. En terminant, je présente un aperçu des propriétés dépressogènes de ces cytokines dans des modèles murins et humains et je propose des hypothèses (et mises en garde) exploratoires quant aux mécanismes par l'entremise desquels les cytokines et les stresseurs peuvent aboutir au trouble dépressif majeur.
Introduction
“What's the matter? You don't seem like yourself today.”
“Yeah. It's nothing. Don't worry about it.”
“Well, something's definitely bothering you.”
“I don't know. I'm just sort of feeling low.”
“Hmm…. Maybe you're coming down with something.”
We've all heard this exchange before, or one like it, and more likely than not it was soon forgotten. Yet, there may be importance to this interaction. Perhaps in the face of an oncoming illness, activation of the immune system gives rise to other biological changes that result in “feeling low.” It has been suggested that such responses are useful, because they encourage individuals to slow down, rest and maintain the resources needed to deal with the oncoming illness.1 In most instances, the cold or influenza will pass, and the ramifications on well-being will be minor. However, what are the consequences of more severe or persistent immunological insults (such as those associated with autoimmune disorders, cancer and even gum disease)? What are the consequences of medical treatments that influence (increase or reduce) immune function? Might immune activation influence anxiety and major depressive disorder (MDD)? If so, through which neurochemical processes does this influence occur?
Few investigators would disagree that stressful experiences are fundamental in the provocation of MDD and other illnesses. Considerable evidence points to a role for stressor-provoked neurochemical changes in the provocation, exacerbation and recurrence of illness. Major depressive disorder has been associated with variations of levels and turnover of serotonin (5-HT), alterations of the 5-HT transporter and several 5-HT receptors (5-HT1A, 5-HT1B, 5-HT2A and 5-HT2C) and dopamine and norepinephrine function.2,3 Moreover, depression has been associated with alterations of peptidergic function, including corticotropin-releasing hormone (CRH), arginine vasopressin, neuromedin B and substance P,4–6 and growth factors (e.g., brain-derived neuro-trophic factor [BDNF]).7,8
In addition to the involvement of conventional processes, there has been a growing appreciation that activation of the inflammatory immune system, particularly the release of immune-signalling molecules (cytokines), might influence many of the neurochemical changes provoked by stressors and contribute to MDD. This position is reinforced by reports indicating that communication occurs among the immune, endocrine, autonomic and central nervous systems so that activation of the inflammatory immune system affects neuroendocrine and central neurotransmitter processes and vice versa.1,9,10 It has been suggested that the immune system operates like a sensory organ informing the brain of antigenic challenge.11 Because the neurochemical changes elicited by inflammatory factors are similar to those elicited by traditional stressors, it has been suggested that the central nervous system might translate immune activation as a stressor.9 Furthermore, by virtue of the stressor-like effects on central nervous system function, inflammatory immune activation might contribute to mood and anxiety-related disorders.9,10,12,13
In the present review, I outline some of the parallels between cytokines and stressors (particularly as they pertain to the development of MDD, variations of monoamine function, alterations of CRH function within the hypothalamic– pituitary–adrenal system and at extrahypothalamic sites) and on variations of BDNF levels. In addition, I review data showing that (a) cytokines and stressors act synergistically in promoting many of the behavioural and neurochemical alterations induced by these treatments and (b) both cytokines and stressors lead to sensitization of neurochemical processes so that later challenges will instigate more pronounced neurochemical disturbances. Finally, based on both animal and human studies, I describe several alternatives concerning the processes by which cytokines and stressors might come to provoke MDD.
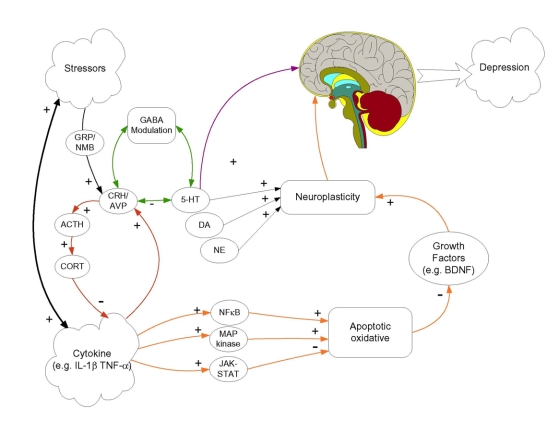
The working model of the mechanisms by which cytokines and stressors may come to promote or exacerbate MDD is depicted in Figure 1.4 Multiple neurochemical alterations may be involved in the provocation of major depression. These neurochemical processes may act in a serial fashion wherein a cascade of neuronal variations is instigated by cytokines and stressors, culminating in MDD. In addition, several factors (e.g., stressors, inflammatory immune activation) may engender parallel neurochemical changes that can act independently, additively or synergistically in promoting MDD. My colleagues and I have also suggested that cytokines and stressors instigate activation of several common neuronal pathways. Among other effects, stressors serving as neurotransmitters and/or neuromodulators activate several neuropeptidergic systems, including neuromedin B, gastrin-releasing hormone and CRH, although CRH variations are not necessarily secondary to the neuromedin B and gastrin-releasing hormone variations. Moreover, CRH changes are not restricted to the hypothalamus, but stressor-provoked variations of this peptide within the amygdala and prefrontal cortex may contribute to the emergence of MDD. These peptidergic processes have been shown to modulate 5-HT function, and it is likely that such effects are moderated by γ-aminobutyric acid receptor A (GABAA) neuronal activity. Furthermore, stressors also affect processes associated with neurogenesis and cell survival, possibly through actions on several growth factors (such as BDNF), which likewise might contribute to MDD.
Fig. 1: Working model showing potential routes by which stressors and cytokines could influence depressive state. Stressors and cytokines may promote several common neurochemical changes, and it is suggested that they may act synergistically in affecting some neurochemical processes. Moreover, stressors and cytokines may result in the sensitization of neurochemical processes, resulting in exaggerated responses to subsequent challenges. Both cytokines and stressors, as depicted in the figure, increase the release of corticotropin-releasing hormones (CRH) in hypothalamic and extrahypothalamic sites, although, in the case of stressors, this outcome may involve activation of bombesin-like peptides (neuromedin B and gastrin-releasing peptide). In addition to activating hypothalamic–pituitary–adrenal function, corticotropin-releasing hormones may influence serotonin (5-HT) processes, and γ-aminobutyric acid receptor A (GABAA) activity may act as a mediator in this regard. The 5-HT variations (as well as those of other amines) may influence depression directly or may do so through other processes. In this regard, an alternative, although not necessarily mutually exclusive, pathway involves cytokine/stress activation of either nuclear factor-κB (NFκB), mitogen-activated protein (MAP) kinases or janus kinase / signal transducer and transcription (JAK-STAT) activator pathway signalling. These would influence oxidative or apoptotic mechanisms, leading to altered growth factor expression (e.g., brain-derived neurotrophic factor [BDNF]), again favouring impaired neuroplastic processes and culminating in major depression. ACTH = adrenocorticotropic hormone; AVP = arginine vasopressin; CORT = cortisol; DA = dopamine; GRP = gastrin-releasing peptide; IL = interleukin; NE = norepinephrin; NMB = neuromedin B; TNF = tumour necrosis factor. Reprinted from Anisman et al.4 Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol 2008;85:1-74, with permission from Elsevier.
As shown in Figure 1, activation of the inflammatory immune system, and especially the release of cytokines (as well as acute phase proteins), may promote many of the neurochemical changes that are elicited by psychogenic stressors. Moreover, stressors themselves may influence inflammatory processes, including cytokine activation. The present paper focuses primarily on the influence of cytokines and stressors on CRH, monoamine and BDNF variations in relation to depression. However, as shown in Figure 1, cytokines also activate other pathways that involve either nuclear factor-κB, mitogen-activated protein kinases, or janus kinase/signal transducer and transcription activator pathway signalling. Activation of these pathways may influence oxidative or apoptotic mechanisms, resulting in variations of growth factor expression such as BDNF, hence promoting MDD. In the present review, I do not describe these latter pathways in any detail because their relation to MDD and several comorbid illnesses (heart disease, Parkinson and Alzheimer disease) have been recently reviewed.4 Finally, there is reason to believe that certain cytokines, through effects on indoleamine-2,3-dioxygenase, may influence 5-HT function and may have neurodegenerative effects, thereby promoting the development of MDD. Although this pathway is not shown in Figure 1, a role for this process is not excluded and is discussed in the present review.
Stressor-provoked neurochemical changes
Analyses of stressor effects in animal models of depression have been useful in defining some of the neurochemical alterations that might subserve pathology. Although most stressors elicit several common outcomes, it appears that the impact of different types of stressors are not entirely congruent. Various processive stressors (i.e., those that require information processing), including both psychogenic and neurogenic insults, do not promote identical effects, nor do conditioned stressors and naturalistic stressors that appear to elicit an innate response (e.g., exposure to predators or related cues).14,15 Human models of depression have shown that certain types of stressors (e.g., anticipation of an aversive event) are particularly likely to provoke anxiety,16 and the types of stressors that have the greatest impact among men may differ from those that have the greatest impact among women. In addition, several very potent stressors in humans such as shame or challenges to self-esteem,16,17 which involve complex appraisal-coping processes, cannot be analyzed in rodent models. Nevertheless, animal-based studies have provided the platform to define the relations between various neuronal systems and some of the moderating variables that influence the effects of stressors. Accordingly, this research has been pertinent to the analyses of the differences encountered in assessing processes related to stressor-provoked MDD.
Monoamine variations
Paralleling the neurochemical changes that are thought to subserve MDD, studies showed that in rodents neurogenic stressors such as foot-or tail-shock influenced the turnover of norepinephrine, dopamine and 5-HT release in several brain regions.18–20 The changes of norepinephrine and 5-HT were particularly marked in specific hypothalamic nuclei (e.g., paraventricular nucleus) and in the hippocampus, amygdala and prefrontal cortex. Likewise, dopamine activity was exceedingly sensitive to stressors and was particularly marked in the prefrontal cortex and the nucleus accumbens.18 Psychogenic stressors similarly increased the release of norepinephrine from mesolimbic neurons9,14,15 and the release of 5-HT from hippocampal neurons.21 The anxiety that accompanied the amine variations was attenuated by chronic antidepressant treatment.22 In vivo, even mild stressors (e.g., tail-pinch, novelty) provoked mesocorticolimbic amine alterations, and the observed effects were pronounced in response to psychosocial stressors.23,24
In addition to altering amine turnover, stressors influence monoamine receptor expression. For example, stressor exposure (forced swim) has been shown to reduce 5-HT1A receptor density in the dorsal raphe nucleus and in the hippocampus, but has the opposite effect in the hypothalamus and amygdala.25 Similarly, it has been reported that behavioural impairments evident after exposure to inescapable shock are accompanied by increased messenger RNA (mRNA) expression of 5-HT1B receptors in the dorsal raphe nucleus, but do not affect postsynaptic 5-HT1B expression in the hippocampus or prefrontal cortex.26
The impact of stressors on neurochemical function is moderated by experiential and organismic factors and by characteristics of the stressors themselves. In the face of an intense stressor whose termination is not within the animal's control, amine release may exceed the rate of synthesis, and stores of norepinephrine and 5-HT begin to deplete.18,27 Under such conditions, animals might be less able to contend with further insults, and behavioural impairments might more likely occur.18,28–30 In a rat study, animals that were first trained in an escapable test (a treatment that immunized them against the behavioural disturbances otherwise engendered by an uncontrollable stressor) did not demonstrate increased 5-HT release at the dorsal raphe nucleus. This outcome was tied to activity of the ventral aspect of the prefrontal cortex, suggesting that this brain region stores and interprets information about stressor controllability.28
It has been suggested that with chronic stressors the adaptive neurochemical systems may become excessively taxed (allostatic overload), leaving animals vulnerable to psychopathology.31 The impact of chronic stressors on monoamine systems is more pronounced when the stressors are unpredictable and when the nature of the stressor varies over days. Likewise, when animals are exposed to a series of stressors that permit adaptive changes to occur, the presentation of a novel stressor leads to augmented amine release.4 With repeated exposure to a stressor, amine synthesis increases and, as a result, the reduction of monoamine levels are not apparent.18 In addition, dopamine use may be moderated with chronic stressors.32 Not surprisingly, these effects are dependent on the nature of the stressors to which animals are exposed, and there is reason to believe that chronic social stressors engender particularly marked and persistent monoamine disturbances.33 With the sustained increase of amine turnover, further downstream effects occur (e.g., downregulation of β-norepinephrine receptor activity and the norepinephrine-sensitive cyclic adenosine monophosphate response,34 reduced 5-HT1B and 5-HT2A receptor expression).35,36,37 Although these neuronal changes might initially protect animals from adverse outcomes, their effectiveness is limited. Moreover, both pathology and allostatic overload are influenced by factors such as the organism stressor history, age and genetic disposition.16
Corticotropin-releasing hormone variations
Stressors instigate changes of several peptides, but there has been a particular focus on the changes of CRH.38 Ordinarily, acute stressors promote the release of CRH at the median eminence, stimulating pituitary adrenocorticotropic hormone and adrenal glucocorticoid secretion.39 However, in contrast to monoamine variations, corticosterone variations do not appear to be markedly influenced by stressor controllability. When a novel stressor is first encountered, it is uncertain whether the stressor is controllable or uncontrollable or whether the stressor will be brief or prolonged. The most efficacious response is one of hypothalamic– pituitary–adrenal activation. As further information regarding the stressor is acquired, neuroendocrine responses might taper off. Thus fast-acting neuroendocrine processes essential for survival would not be expected to be influenced by stressor controllability.4 As the stressor continues, differences between the hypothalamic–pituitary–adrenal effects of controllable and uncontrollable stressors might emerge.
Stressor-provoked CRH alterations are not restricted to the hypothalamus; variations of in vivo release and CRH mRNA expression have been observed in the central amygdala.40,41 However, the processes involved in promoting the latter effects are distinct from those associated with hypothalamic CRH variations. In contrast to the inhibition of hypothalamic CRH associated with the corticosterone (or cortisol) stimulation of hippocampal receptors, in the central amygdala and bed nucleus of the stria terminalis, cortisol increases CRH expression and GABAA neuronal function, thus provoking anxiety.42,43
Diffuse stimuli that promote general anxiety are thought to be associated with CRH variations in the bed nucleus of the stria terminalis.44 In contrast, the increase of CRH expression in the central and basolateral amygdala may be fundamental for the acquisition (rather than the expression) of a fear response.44,45 Although a range of stressors influence in vivo CRH release in the amygdala, the effectiveness of CRH antagonists in attenuating anxiety is dependent on the nature of the stressor to which animals were exposed.15,41 Unlike anxiety elicited by neurogenic stressors (or cues explicitly paired with a stressor), CRH antagonists do not alter the anxiety provoked by naturalistic stressors (e.g., predators), which may involve prewired neural circuits.
In line with CRH involvement in anxiety and depression, the CRH antagonist CP-154 526 attenuated the behavioural disturbance (learned helplessness) elicited by uncontrollable shock.46 In a study of genetically engineered mice with elevated or reduced CRH receptors, predictable alterations of anxiety and depressive-like behaviour were apparent.47 Essentially, anxiety was elevated among mice that overproduced CRH, although this outcome was specific to the behavioural test used.48
There is also evidence that the type 1 CRH receptor (CRH1) may be involved in anxiety and depression. It has been reported that CRH1 receptor expression is reduced in the prefrontal cortex in chronically stressed animals, a condition akin to the sustained distress of severe depression.49 Conversely, anxiety levels are diminished in CRH1 receptor knockout mice,50–52 although this outcome is dependent upon the basal levels of emotionality that were ordinarily apparent.53 Furthermore, among conditional knockout mice (Crhr1(loxP/loxP)Camk2a-cre) in which CRH1 receptors are inactivated in the anterior forebrain and in limbic brain regions (without affecting pituitary receptors), anxiety is reduced. In contrast, elevated CRH1 receptors were associated with a greater response to stressors.54 Although these data support the view that CRH1 variations may be pertinent to the development of anxiety, it ought to be considered that subtypes of anxiety (state v. trait, contextual or stimulus-bound) may involve different aspects of CRH function.
The involvement of CRH2 in anxiety is less clear, although it has been reported that knocking out this receptor subtype increases anxiety-like behaviours,55 most notably among male mice.56 Given the independent effects of CRH1 and CRH2 variations, Preil and colleagues57 assessed corticosterone and adrenocorticotropic hormone under basal conditions and in response to social defeat among double and single CRH1 and CRH2 knockout mice. In the CRH1 knockout mice and in the double knockout mice, the corticosterone and adrenocorticotropic hormone responses associated with stressors were attenuated, and the presence of the CRH2 gene did not compensate for the absence of the CRH1 gene, implicating the primacy of the CRH1 receptor. However, elevations of anxiety were more prominent among male mice than among females. Although these data point to CRH involvement in depression, it is unclear why CRH variations have a greater effect in male than in female mice, whereas anxiety and depression in humans are more frequent among women than men. At this time, it is uncertain whether the relative density and sensitivity of CRH2 receptors in men and women differ from those in mice. Furthermore, it is unlikely that CRH or CRH receptors are alone in affecting anxiety or depression; other factors might be responsible for the sex differences observed in humans.
Brain-derived neurotrophic factor variations
In line with the possibility that BDNF is associated with MDD, reductions of BDNF could be induced by psychological stressors (predator exposure and explicit or contextual cues previously paired with foot-shock)58,59 and by chronic stressors.60 Elevated anxiety in mice has been associated with reduced BDNF in the hippocampus,61 and BDNF infusion into the dorsal raphe nucleus62 or the hippocampus63 provokes antidepressant-like behavioural effects. Furthermore, antidepressant therapy increases BDNF expression and tropomyosin-related kinase B signalling, and it attenuates the effects of stressors.64–66 In a forced swim test, the antidepressant actions of desipramine were attenuated among BDNF knockout mice, pointing to the essential role of this trophic factor in accounting for the effects of antidepressant therapy.67 Interestingly, BDNF expression seems to be dependent on the estrus cycle and could be reduced by exogenous estradiol therapy, as estradiol has been shown to attenuate the upregulation of BDNF elicited by a 5-HT2A agonist.68
Consistent with the view that BDNF may be associated with MDD, it has been reported that BDNF expression and protein levels, and that of its tropomyosin-related kinase B receptors, are lower within the prefrontal cortices and hippocampi of individuals who die by suicide than in age-and sex-matched controls.69 In addition, BDNF levels are reduced in depressed patients and are inversely correlated with the degree of clinical impairment and reductions of hippocampal volume. With successful antidepressant therapy, BDNF serum levels normalize.70–72 The relation between BDNF and depression may be sex-dependent. In particular, reduced BDNF levels are notable in depressed women but not men, and chronic antidepressant treatment selectively elevates the growth factor in women.72 Therefore, it is possible that the more frequent appearance of depression in women might be related to interactions between sex hormones, 5-HT receptors and BDNF.
Stress, cytokines and depression
A brief primer on cytokines
Immune cells (T and B lymphocytes, macrophages and endothelial cells) synthesize and secrete cytokines that function as signalling molecules. These cells also elicit growth and differentiation of lymphocytes. I will focus on the proinflammatory cytokines, namely interleukin-1β, interleukin-6, tumour necrosis factor-α and interferon-α, because they have received the greatest attention with respect to depressive illness. However, it should be noted that other cytokines (especially anti-inflammatory cytokines such as interleukin-4 and interleukin-10) might influence immune–brain interactions and their effects on psychopathology.
In the case of interleukin-1β, signalling involves stimulation of type 1 receptors73 that are found on the surface of lymphocytes, macrophages or monocytes, neurons and glial cells within the brain. Regulation of interleukin-1β involves an endogenous antagonistic ligand (interleukin-1β receptor antagonist) that competes with interleukin-1 for binding sites,74 and the anti-inflammatory cytokines interleukin-4 and interleukin-10. After activation of the interleukin-1β receptor, a cascade of changes ensue, culminating in the phosphorylation and degradation of the endogenous inhibitory factor-κB. This leads to the translocation of nuclear factor-κB to the nucleus, where it promotes the expression of several genes that influence neuronal homeostasis and inflammatory processes.74
In addition to interleukin-1β, macrophages (and microglia within the brain) also produce tumour necrosis factor-α, which may affect mononuclear and polymorphonuclear blood cells, fibroblasts, keratinocytes, insulin-sensitive adipocytes, brain microglia and neurons. Ordinarily, endogenous antagonists for tumour necrosis factor-α, including α-melanocyte–stimulating hormone, block the actions of tumour necrosis factor-α and contribute to its regulation.75 Tumour necrosis factor-α stimulates 2 distinct receptor subtypes: p55 and p75. The p75 receptor contributes to nutritive functions such as the promotion of thymocyte proliferation, whereas the p55 receptor is important in apoptosis and cellular necrosis.76 Tumour necrosis factor-α possesses a unique apoptotic signalling mechanism involving a death domain region whose activation triggers the recruitment of caspases 1 and 8, thereby provoking cytotoxic actions.77 Several intracellular actions of the p55 receptor may be counterbalanced by the p75 receptor, which may under some circumstances have inhibitory effects on apoptosis.78
Macrophages and T lymphocytes also secrete interleukin-6, which stimulates receptors on other immune cells, microglia, astrocytes, neurons and cells such as fibroblasts, endothelial cells and granulocytes that are not typically considered to be part of the immune system.74,79 The recruitment of glycoprotein-130 is necessary for functional binding of interleukin-6 to its receptor, and the resulting complex leads to a signalling cascade that involves activation of the janus kinase / signal transducer and transcription activator pathway. Subsequent nuclear translocation of the pathway's homo-or heterodimers provokes the transcription of several immune and central nervous system regulatory proteins.80
Finally, interferons can be divided into either type 1 interferons, including the interferon-α and interferon-β isoforms, and the structurally unrelated type 2 interferon-γ, which shares several functions with other interferons. Like interleukin-6, the signalling pathway used by interferons is the janus kinase / signal transducer and transcription activator pathway, resulting in sequential phosphorylation steps by intracellular protein kinases.81 The type 1 interferons also stimulate phosphatidylinositol 3–kinase and mitogen-activated protein kinase pathways that are responsible for the ability of this cytokine to interfere with viral replication and the promotion of apoptotis of tumour and other cells.82
Owing to their large size, cytokines do not readily enter the brain, although limited access is possible at sites where the blood–brain barrier is relatively permeable such as the circumventricular organs (e.g., subfornical organ, area postrema, organum vasculosum). In addition, entry of interleukin-1β and tumour necrosis factor-α into the brain can occur through saturable transport mechanisms,83 and actions of cytokines on central nervous system processes may occur indirectly through the stimulation of visceral branches of the vagus nerve.1,84 However, proinflammatory cytokines may influence blood–brain barrier permeability and upregulate their own production at vascular sites.85 In addition, chemokines (chemo-attractant cytokines) can also disrupt blood–brain barrier function and may be responsible for the vasogenic brain edema that often accompanies brain trauma, ischemia, central nervous system infection, presence of brain tumours and recurrent multiple sclerosis flares.86 Stressful events and immunologic challenges may also compromise the integrity of the blood–brain barrier, thereby influencing entry of cytokines into the brain parenchyma.87
Within the brain, cytokines interact with endogenous receptors present on cells lining the blood–brain barrier and vascular areas.88,89 Moreover, through volume diffusion, cytokines may reach hypothalamic, amygdaloid and brain stem nuclei where interaction with specific receptors may occur,90,91 activating intracellular second messenger systems and stimulating mitogen-activated protein kinase pathways.
In addition to the fact that peripherally produced cytokines activate central processes through direct and indirect actions, cytokines, their receptors and anti-inflammatory factors are also endogenously expressed in the brain, being produced by glia and possibly by neurons.88,91 Messenger RNA expression of these cytokines (and their protein levels) are increased by endotoxins administration, traumatic insults, head injury, stroke and neurotoxins.92–95 Moreover, stressors increase cytokine mRNA expression and protein levels in the brain,94,96,97 and it has been suggested that neuroinflammatory cascades in the brain may contribute to neurologic and neuropsychiatric conditions.4
Cytokines engender stressor-like effects
Not so long ago, it was thought that the actions of the immune system were independent of central nervous system function. However, it is now clear that activation of particular brain sites (and stimuli such as stressors that promote increased central neuronal activity) influence immune activity. Conversely, immune activation may influence various hormones and central neurotransmitter activity.4,11,98 The overview that follows shows that cytokines (or immune activation elicited by endotoxins), through their actions on monoamines, CRH and BDNF might contribute to the development of MDD.
Proinflammatory cytokines increase the release of CRH from the median eminence, leading to pituitary adrenocorticotropic hormone and adrenal corticosterone release.9,10,99 In addition to these hypothalamic–pituitary–adrenal effects, like psychogenic and neurogenic stressors, cytokines also influence CRH activity at the central amygdala100,101 and increase the use of 5-HT and norepinephrine within the paraventricular nucleus of the hypothalamus (paraventricular nucleus), prefrontal cortex, hippocampus and the central amygdala.9,102,103 Such effects have been observed in postmortem tissue and in vivo, and they are apparent when the cytokine is administered systemically or centrally.104–109 Although interleukin-1β has been the object of the greatest attention, both interleukin-6 and tumour necrosis factor-α have been shown to affect central monoamine activity, although their actions are less pronounced than those provoked by interleukin-1β.9,108–110
In addition to their potent individual actions, interleukin-1β plus interleukin-6 and interleukin-1β plus tumour necrosis factor-α have been shown to synergistically increase behavioural (sickness behaviours and reduced consumption of highly palatable substances) and hypothalamic– pituitary– adrenal alterations.111–114 When interleukin-1β and interleukin-6 are administered together, the adrenocorticotropic hormone response is elicited earlier than that provoked when either of these cytokines are administered alone.115 Just as synergies involving different cytokines have been observed, when a mild stressor was administered in rats that had been treated with interleukin-1β, the in vivo release of 5-HT from the hippocampus was greatly increased.105 More recently, it has also been observed that when an immune challenge (e.g., interleukin-1β, lipopolysaccharide or the viral imitator, poly I:C) is administered against a psychosocial stressor backdrop (e.g., reuniting cage mates after a period of separation), the behavioural (sickness), corticosterone and central norepinephrine alterations ordinarily elicited by immune challenges are greatly increased. The immune challenges also increase the circulating levels of both pro-and anti-inflammatory cytokines, and they markedly increase their mRNA expression in hypothalamic and extrahypothalamic brain regions.116–118 Because viral and bacterial challenges concurrently or sequentially affect multiple cytokines (and other inflammatory factors), and because MDD likely involves several neurochemical disturbances, the broad synergistic actions associated with cytokines and stressors may be particularly important in the development of MDD.
Consistent with the involvement of cytokines in the stress response, it has been reported that inhibition of inflammatory cytokine function could attenuate the effects ordinarily provoked by stressors. Specifically, the stressor-provoked hypothalamic monoamine alterations ordinarily elicited by a stressor are precluded if rats are pretreated with the interleukin-1β antagonist (interleukin-1 ra).107 Likewise, the behavioural and neuroendocrine effects of a chronic mild stressor, and impaired hippocampal neurogenesis, are inhibited among interleukin-1β receptor knockout mice.119 These data implicate interleukin-1β in the circuitry mediating the monoaminergic effects of a stressor, but this interrelationship may be specific only to some cytokines. Although the corticoid elevation elicited by tumour necrosis factor-α is greatly attenuated in tumour necrosis factor-α p55 receptor knockout mice, the hypothalamic–pituitary– adrenal response provoked by restraint or lipopolysaccharide is unaffected in these mice.4
Unlike the burgeoning literature indicating that stressors reduce BDNF in the brain, there have been few reports concerning the relation between cytokines and BDNF. However, it has been shown that interleukin-1β increases BDNF mRNA expression in hypothalamic neuron-enriched cultures. An opposite effect was apparent in mixed neuron-astrocyte cultures.120 This suggests selectivity regarding the site of cytokine actions (neuronal v. glial). It has also been reported that tumour necrosis factor-α increases BDNF expression in primary astrocytes, apparently through actions on nuclear factor-κB,121 and that interleukin-6 and tumour necrosis factor-α are involved in the regulation of BDNF secretion in monocytes.122
Consistent with the view that cytokines may influence anxiety and depressive states through actions on BDNF, it has been reported that both interleukin-1β and tumour necrosis factor-α reduce BDNF expression in the amygdala.123 In addition, interleukin-1β disrupts BDNF-induced signal transduction mediated by tropomyosin-related kinase B and interferes with the protection of neurons from apoptosis.124 These findings suggest similarities between the effects of stressors and cytokine administration on BDNF variations, just as they have congruent actions on proinflammatory cytokines. Attributing the depressogenic actions of cytokines to variations in BDNF is speculative but, nevertheless, warrants further consideration. There are reports that do not comfortably fit within this framework. Although stressors and cytokines might be expected to have parallel effects, it has been reported that fear conditioning in rats is associated with an increase of BDNF production in some portions of the hippocampus (possibly reflecting the learning of the fear response) and that this outcome is eliminated by interleukin-1β;125 the reasons for this are unclear. As indicated earlier, the elevated BDNF may be related to the rats learning the fear response, and it is possible that unpredictable, uncontrollable stressors (or chronic insults) would have yielded different results.
To this point, the discussion of cytokine effects has focused primarily on interleukin-1β, interleukin-6 and tumour necrosis factor-α. Even though interferon-α has found its way into the clinical treatment of particular forms of cancer and hepatitis C, the behavioural, hormonal and central neurochemical consequences of this cytokine have yet to be explored fully. Under normal conditions, systemic administration of interferon-α elicits modest behavioural changes,117 but it has been reported that when interferon-α is administered directly into the brain, fever, anorexia and analgesia are elicited, as are changes of hypothalamic neuronal firing.126 Like other cytokines, interferon-α increases plasma cortisol levels, but the magnitude of increase is small in comparison to that provoked by interleukin-1β.116 It has also been observed that, at low doses, interferon-α reduces corticosterone, but when administered directly into the brain it increases circulating corticosterone levels.127 In vitro, interferon-α stimulates CRH release within cells of the amygdala and hypothalamus128 and provokes in vivo neurochemical and hormonal changes in the brain.129
In addition to hypothalamic–pituitary–adrenal effects, systemically administered interferon-α stimulates GABA and glutamate function within limbic and hypothalamic regions,130 increases 5-HT transporter mRNA expression,131 modestly increases hypothalamic and hippocampal norepinephrine use116 and reduces dopamine concentrations within the amygdala.132 In addition, when interferon-α is administered repeatedly, dopamine use133 and 5-HT levels in the prefrontal cortex are reduced,134 and 5-HT turnover increases within the amygdala.135 Consistent with the position that inflammatory processes are associated with the actions of interferon-α, it has been shown that the reduced 5-HT turnover in the prefrontal cortex and the depressive-like behavioural effects of the treatment are attenuated in rats that have been pretreated with the nonsteroidal anti-inflammatory drug diclofenac.134,135 Interferon-α also promotes 5-HT2C receptor mRNA editing, resulting in receptor downregulation.136 This outcome may be particularly relevant, because 5-HT2C receptor function has been associated with anxiety and depressive symptoms,137 and because altered 5-HT2C editing has been associated with depression and suicide.138
As in animals, acute interferon-α treatment increases adrenocorticotropic hormone, cortisol and circulating interleukin-6 in healthy human volunteers and in patients with hepatitis.139,140 However, with repeated interferon-α therapy, the elevated hypothalamic–pituitary– adrenal activity is not apparent.141 Furthermore, in mice, the sickness associated with interferon-α diminishes over 5 successive days of treatment; however, the corticosterone elevations are moderately increased relative to acute treatment.116
Sensitization effects
Beyond their immediate effects, stressors may proactively influence neurochemical responses to subsequent stressors (i.e., sensitization). The sensitization of neuronal processes might be a key factor in promoting later stressor-provoked pathology and the very high rate of illness recurrence that is characteristic of depression.9,19,142 With successive stressor experiences (or with repeated bouts of depression), the degree of distress necessary to promote a further episode declines so that even minor events may be sufficient to precipitate a depressive episode.143,144 Furthermore, sensitized neuronal changes may be tied to anxiety processes, as the amine changes elicited by stressor re-exposure or stressor-related cues can be attenuated by anxiolytics and 5-HT1A receptor manipulations.145
Among previously stressed animals, later application of a mild stressor may result in augmented norepinephrine and dopamine in the prefrontal cortex and hippocampus and augmented 5-HT use within the medial prefrontal cortex, and this occurs even if the 2 experiences involve very different types of challenges (cross-sensitization). Likewise, increased monoamine use is apparent when the second challenge involves a pharmacological stimulant such as amphetamine or cocaine.142 Moreover, in addition to altering amine levels and turnover, stressor re-exposure enhances hippocampal 5HT1A receptor density while decreasing the affinity of those receptors. In contrast, 5-HT2A receptor density is unaffected, whereas its affinity increases.146
The stressor-induced sensitization of dorsal raphe 5-HT neuronal activity, which has been implicated in depression and anxiety, may involve a ligand related to CRH2 receptors,147 whereas the cross-sensitization between stressors and amphetamine may involve N-methyl-D-aspartate–glutamatergic receptors.148 Intriguing data have also been reported suggesting that basic fibroblast growth factor, a neurotrophic factor produced by astrocytes, may be fundamental for the neuronal plasticity associated with the protracted effects elicited by stressors and catecholamine stimulants.149 Evidently, varied neuronal systems are subject to sensitization and thus might contribute to varied pathological outcomes.
Cytokines, like stressors, promote greater neuroendocrine and neurotransmitter responses when animals are re-exposed to the cytokine.142 Both tumour necrosis factor-α and lipopolysaccharide may influence the coexpression of CRH and arginine vasopressin,100,150 just as interleukin-1β has previously been shown to promote this effect.151 It has been reported that paraventricular nucleus neurosecretory neurons have the capacity for phenotypic plasticity so that, among animals that were stressed or treated with cytokine, increased arginine vasopressin occurred within terminals of the external zone of the median eminence that contain CRH. Upon release, these secretagogues synergistically influence pituitary adrenocorticotropic hormone release, and, upon later exposure to the same cytokine or to a stressor, greater hypothalamic–pituitary–adrenal activity is provoked.99,151,152 These effects become more pronounced with the passage of time (effects were marked 14–28 d after treatment, but minimal after 1–7 d). Just as cytokines increase the later response to stressors, it has also been shown that the response to a cytokine challenge is enhanced among rats that have previously been stressed.149 However, if rats are pretreated with an interleukin-1 antagonist, then the effects of the subsequent stressor are attenuated.153
The sensitization associated with stressor and cytokine treatments is also evident within the amygdala, although the nature of the outcome is distinctly different from that seen in the hypothalamus. Systemic tumour necrosis factor-α administration results in the sensitization of CRH-related processes in the central amygdala so that later tumour necrosis factor-α challenge greatly augments CRH immunoreactivity. Unlike the median eminence changes, the elevated CRH immunoreactivity occurs as early as 1 day after the initial treatment and is only apparent upon re-exposure to the cytokine.100 Moreover, given the paucity of arginine vasopressin in this site, it seems that some mechanism other than the coexpression of CRH and arginine vasopressin is responsible for the sensitized neuronal responses.
Although it might be expected that cytokine-related behaviours would be subject to sensitization-like effects, there is currently limited information about behavioural changes that occur upon re-exposure to a cytokine or upon exposure to a stressor in animals previously treated with a cytokine. Even less information is available on the effects of stressor or cytokine re-exposure in animal models of depression (i.e., those that analyze anhedonia rather than motor activity or sickness) or about processes that have been linked to depression, including 5-HT receptor changes, 5-HT transporter or BDNF function. However, it has been reported that interleukin-1β treatment does not augment the shuttle escape deficits otherwise provoked by later interleukin-1β therapy.154 In contrast, there has been suggestive evidence of sensitization associated with inflammatory immune changes reported after child birth among women with a lifetime history of depression.155
A brief digression is appropriate at this point, given that inflammatory responses engendered by early life events may have proactive effects, just as positive or negative experiences in early life may influence the adult response to different types of challenges.156 It has been shown that early-life exposure to inflammatory challenges affects adult responses to stressors, reflected by exaggerated corticosterone reactivity, elevated lymphocyte sensitivity to suppression and protection against adjuvant-induced arthritis.157 Although these data do not necessarily suggest common mechanisms for the sensitization in adults who experienced stressors early in life, they do illustrate the protracted impact of immunogenic stressors, particularly in the context of later vulnerability to pathology.
Behavioural effects of cytokines in animal studies
As indicated earlier, the immunologic and neurochemical changes provoked by inflammatory challenges likely facilitate adaptive responses to meet ongoing demand.1,90,158 The sickness associated with inflammatory immune activation may maintain energy resources to deal with the systemic insult.1 Conveniently, under conditions in which behaviours associated with sickness might be disadvantageous (e.g., in a novel or threatening environment), the expression of these behaviours may be suppressed.159 Yet it is clear that, in association with immune activation, behavioural changes that favour adverse outcomes, particularly the promotion of anxiety and depression, are instigated.10
In rodents, immune activation provoked by bacterial endotoxins (e.g., lipopolysaccharide) and administration of interleukin-1β or tumour necrosis factor-α provoke soporific effects, ptosis, anorexia, fever, fatigue, reduced motor activity and curled body posture, which have collectively been referred to as “sickness behaviours.”90 In addition, these treatments disrupt exploration, social interaction and operant responding for food reward,90,160 which may be secondary to the sickness behaviours. Because central administration of low doses of interleukin-1β or tumour necrosis factor-α also provoke signs of sickness, these behaviours are likely mediated through central nervous system processes.90,161
Inasmuch as antidepressant therapy attenuates sickness behaviours162 and the elevated Fos expression163 provoked by endotoxins, it has been suggested that the sickness behaviours represent symptoms of depression. Further to the view that inflammatory processes contribute to depression, it has been reported that cyclooxygenase-2 antagonists (nonsteroidal anti-inflammatory drugs) diminish the depressive-like behavioural and neurochemical alterations elicited by lipopolysaccharide and interferon-α administration,134,164 limit the corticosterone response to social stressors165 and limit the effects of lipopolysaccharide on Fos immunoreactivity within 5-HT neurons.166
Although sickness behaviours are not characteristic of the primary symptoms of depression, they may reflect neurovegetative features associated with MDD (e.g., decreased appetite, motor retardation).10 It is equally likely that these “symptoms” are a reflection of general malaise rather than depressive illness. Thus it is particularly important that cytokines and bacterial endotoxins also seem to elicit anhedonia, a key symptom of depressive illness, independent of sickness (reflected by anorexia).9,167–169 It has been shown that when rats are tested in an operant task using a progressive ratio schedule for sucrose, performance is disrupted by interleukin-1β. In the progressive ratio test, the number of operant responses required for a fixed amount of sucrose increases progressively with each reward. This test provides an index of the motivation to work for reward in the face of increasing costs. In the test my colleagues and I used,9 animals were on a progressive ratio schedule to obtain a sucrose reward, but they also had free access to standard laboratory chow. Although free chow consumption and progressive ratio performance for sucrose were both diminished after interleukin-1β administration, recovery from the effects of the cytokine differed for free chow and for sucrose. Moreover, chronic antidepressant therapy attenuated the disruption of progressive ratio performance for the sucrose, but did not attenuate the cytokine's effect on free chow consumption. These data not only support the view that interleukin-1β may instigate depressive-like effects, but also indicate that the anhedonia was independent of effects on anorexia.169,170 It has also been shown that treatment with an endotoxin (lipopolysaccharide) reduces responding for rewarding brain stimulation (using a current titration schedule). In this test, rats that receive lipopolysaccharide will and can respond to obtain rewarding brain stimulation, but they more readily stop responding once the value of the responses (delivery of a low current) is sufficiently diminished.171
Impact of chronic cytokine treatment
Studies about the influence of cytokines on neurochemical and behavioural processes have typically evaluated the impact of acute treatments. Inasmuch as viral insults and cytokine immunotherapy all involve relatively persistent cytokine elevations, greater attention to the impact of chronic challenges is clearly needed (although the cost of cytokines may be prohibitive). There is reason to believe that chronically increased cytokine levels may engender particularly profound effects, possibly owing to the potential for compromised blood–brain barrier function. In addition, chronic cytokine elevations acting as a stressor may exert excessive strain on physiologic systems, just as chronic exposure to stressors might have such an effect.31 In fact, sustained systemic or intracerebroventricular interleukin-1β administration promotes persistent hypothalamic–pituitary–adrenal activation, including protracted variations of CRH, CRH receptor and proopiomelanocortin gene expression, elevated secretion of adrenocorticotropic hormone, β-endorphin and corticosterone.172,173 Furthermore, chronic central or intravenous interleukin-1β therapy increases immediate early gene expression in the paraventricular nucleus, central amygdala and supraoptic nucleus.174 As expected, anxiety and depressive characteristics in rats are associated with protracted interferon-α treatment, and the anhedonic effects of the cytokine only emerge after rats have received 2 weeks of treatment.175
Behavioural correlates of cytokines in human studies
Human studies have shown that MDD is associated with elevated levels of circulating proinflammatory cytokines (and mitogen-stimulated cytokine production) and their soluble receptors as well as with indications of an acute phase response (e.g., haptoglobin, α1-antitrypsin, α1- and α2-macroglobulin and C-reactive protein) — all evidence that immune activation accompanies depression.12,13,176 In fact, the range of cytokines that is altered in patients with MDD compared with controls is exceedingly broad (macrophage inflammatory protein-1α; membrane cofactor protein-1; interleukin-1α, 1β, 2, 4, 6, 7, 8, 10 and 15; granulocyte-macrophage colony-stimulating factor; interferon-γ), and some of these cytokines have not typically been linked to depressive illness.177 Although some studies show that the elevated levels of interleukin-1β, interleukin-6, tumour necrosis factor-α and interferon-γ associated with MDD are normalized with antidepressant therapy,178–180 other studies have not supported this finding.13,181 However, relative to other cytokines, interleukin-6 changes are more readily provoked with antidepressant therapy. It has been shown that depressed patients who respond positively to treatment with escitalopram have lower levels of tumour necrosis factor-α than do nonresponders.182 Overall, there seems to be some question as to whether cytokine elevations are simply markers of depression or whether they are causally related to the illness. It is possible that normalization of cytokine levels requires sustained treatment with antidepressants, that normalization only occurs in a subset of patients and that failure of cytokine normalization is a harbinger for illness recurrence.
Studies that support a relation between cytokine levels and MDD have not necessarily excluded a role for factors secondary to MDD in promoting the elevated cytokine levels. Hence the meaning of the cytokine–depression relations are uncertain. It has been suggested that factors that lead to depression (e.g., recent stressful experiences or those experienced early in life) might influence cytokine levels.4 Similarly, institutionalization (and the accompanying social and environmental change), drug use (including nicotine, alcohol and illicit drugs), eating habits or general care may influence cytokine levels.183 When cytokine levels are assessed controlling for age, body mass index, sex, smoking habits, recent infection or prior medication, differences of circulating cytokine levels between controls and depressed patients are absent.184 It is also possible that neurovegetative features (e.g., reduced appetite, weight loss, decreased sleep) evident in typical major depression could promote altered cytokine function; altered circadian cycles that could occur among depressed people might also influence cytokine production.185 Finally, MDD may be comorbid with a variety of other illnesses, including heart disease, diabetes and Parkinson disease,4 any of which could directly or indirectly influence circulating cytokine levels.
Despite the limitations of the correlational studies, when coupled with case–control studies, a relatively strong case can be made for inflammatory immune activation in MDD. It has been shown that immune-activating agents (e.g., endotoxin treatment or vaccines) administered in healthy adults may elicit depressive-like behaviours in direct relation to tumour necrosis factor-α and interleukin-6 secretion.186,187 Furthermore, there have been a large number of studies showing that immunotherapy (using interferon-α to treat some forms of cancer and hepatitis C) may lead to marked depressive illness.188–197 In some instances, these symptoms are sufficiently severe to necessitate termination of the therapy.198 Although depressive symptoms ordinarily resolve with treatment cessation, they are likely to reappear with subsequent cytokine continuation.199 However, it has also been reported that a second round of treatment is not accompanied by exacerbation of psychiatric symptoms.200
The development of depression associated with interferon-α therapy is also influenced by factors that predispose to depression under other circumstances. For example, subsyndromal levels of depression before immunotherapy predict MDD in response to interferon-α,201,202 as do poor social support resources.202 Furthermore, as observed in relation to mood changes elicited by lowering the 5-HT precursor tryptophan,203 susceptibility to depressive symptoms induced by interferon-α are greater among women and patients with a history of depression.204,205 Moreover, tryptophan levels are predictive of depressive illness,204 as are the initial elevations of adrenocorticotropic hormone and cortisol in response to interferon-α.191 Finally, patients with the highest baseline levels of s-interleukin-2r, interleukin-6, and interleukin-10, are particularly prone to depressed mood following immunotherapy.206 Although there has been some question as to whether the sickness associated with cytokine treatment actually reflects depression, vegetative symptoms after 4 weeks of interferon-α therapy predict the occurrence of subsequent MDD.207
Together, these data not only provide support for cytokine involvement in the provocation of depression, but also support the view that altered 5-HT function caused by interferon-α therapy may be fundamental in eliciting this outcome. Indeed, there have been several reports indicating that selective serotonin reuptake inhibitors (e.g., sertraline, paroxetine, citalopram) diminish or eliminate the depressive symptoms provoked by interferon-α.194,208–211 There have also been indications that selective serotonin reuptake inhibitors could be used prophylactically to limit the emergence of symptoms,208,212,213 although paroxetine, for instance, has been shown to be ineffective in this capacity.214 Interestingly, as observed in animal studies assessing the effects of antidepressants in attenuating the effects of interleukin-1β, it also appears that paroxetine primarily affects the mood-related symptoms and has only minor effects on sickness-like behaviours, including fatigue and anorexia.215,216 Further support for inflammatory involvement in depression has come from studies showing that a cyclooxygenase-2 inhibitor enhances the effectiveness of antidepressant medication in treating depression in humans,217 although the practical use of this procedure is limited by the risk of gastrointestinal disturbances that may be provoked by anti-inflammatory agents, particularly when used in conjunction with selective serotonin reuptake inhibitors.218
The finding that selective serotonin reuptake inhibitors attenuate the depressogenic actions of cytokines is congruent with the position that 5-HT alterations are fundamental to at least some of the adverse actions of proinflammatory agents. However, this does not belie the possibility that selective serotonin reuptake inhibitors may act through other processes. Fluoxetine has been shown to reduce interleukin-1β, interleukin-6, interferon-γ and tumour necrosis factor-α expression while increasing circulating levels of the inhibitory cytokine interleukin-10.219 Desimipramine reduces tumour necrosis factor-α levels within the hippocampus and brainstem,220 and the clinical efficacy of this compound has been linked to its ability to alter the sensitivity of norepinephrine neurons to tumour necrosis factor-α.221 Pretreatment with paroxetine has been shown to prevent the elevated interleukin-1β in blood and in the hippocampus induced by interferon-α.222 In fact, acting through different processes, a variety of antidepressants (e.g., amitriptyline, clomiprimine, imipramine, bupropion) all diminish interleukin-1β and tumour necrosis factor-α release provoked by lipopolysaccharide.223,224 Yet another class of compounds with antidepressant effects, the phosphodiesterase IV inhibitors (e.g., rolipram), reduce cytokine production in response to immune activation.225,226 When considered as a whole, the studies assessing the effects of selective serotonin reuptake inhibitors (and other antidepressants) indicate that inflammatory factors contribute to cytokine-induced depression, but it is uncertain whether the effects observed are necessarily attributable to 5-HT variations.
Despite the impressive evidence showing that interferon-α immunotherapy elicits a depressive-like state, several caveats need to be stated concerning the behavioural actions of this cytokine. The effects of interferon-α are not limited to engendering depressive symptoms. In fact, patients treated with interferon-α present with cognitive disturbances, impaired concentration and memory, irritability, and manic periods.198,227,228 Moreover, at high doses, interferon-α may provoke a confused state characterized by disorientation and psychotic-like features.216 Cognitive impairments (e.g., disturbances of immediate recall) may become increasingly pronounced over the course of the treatment regimen.216,229 In addition, interferon-α immunotherapy may provoke disturbed vigilance, alertness and some memory problems, which diminish with treatment cessation. Thus, despite the positive effects of antidepressant treatments, the nonspecificity of symptoms associated with interferon-α immunotherapy begs the question: are the effects elicited by immunotherapy a genuine manifestation of MDD or are they a reflection of general malaise or toxicity?230 This question notwithstanding, the emergence of depressive symptoms associated with interferon-α immunotherapy appears to be independent of cognitive disturbances. In fact, whereas 82% of their sample developed depressive symptoms, Reichenberg and colleagues186 found that 30% of patients experienced cognitive problems.
Another factor that warrants consideration is that the effects of interferon-α have not been assessed in healthy individuals, but in patients undergoing considerable strain (related to cancer or hepatitis C). Thus it is conceivable that the depressive effects associated with interferon-α immunotherapy actually represent the actions of the cytokine being superimposed on a stressful background. In fact, in animals, acute interferon-α elicits only modest behavioural effects (e.g., limited sickness), corticoid variations and effects on central monoamine function. However, when an acute interferon-α challenge is coupled with a potent social stressor (reuniting cage mates after a period of isolation, thereby promoting re-establishment of social hierarchies), the behavioural, neuroendocrine and central norepinephrine variations otherwise provoked by interferon-α are markedly enhanced.117
Mechanisms subserving cytokine-induced depression
As indicated earlier, cytokines may promote depressive states through any of several different neuronal processes (and these are not mutually exclusive). Cytokines such as interferon-α stimulate indoleamine-2,3-dioxygenase and GTP cyclohydrolase I activity. This activity, in turn, promotes degradation of the 5-HT precursor tryptophan, thereby reducing 5-HT function and increasing vulnerability to depressive illness.10,188,196,204 In addition, indoleamine-2,3-dioxygenase promotes the catabolism of tryptophan into kynurenine and then into the oxidative metabolites 3-hydroxy-kynurenine and quinolinic acid (which themselves are increased in depression). These metabolites may have neurotoxic effects through the induction of free radical generation196,197 and hence may contribute to neurodegeneration and MDD.4,231
Although indoleamine-2,3-dioxygenase involvement in promoting depression has not been excluded,19 it has been suggested that MDD associated with inflammatory immune activation may stem from a cascade of neurochemical and growth factor changes.4 Like stressors, cytokines could potentially influence major depression through 2 major routes that each feed into several interconnected loops. Stressors and cytokines increase both hypothalamic and extrahypothalamic CRH release, which may come to provoke altered 5-HT function, possibly being moderated by GABAA activity. This, in turn may influence depression directly or may do so by impairing neuroplastic processes involving BDNF (or other growth factors). Although the present review has focused on this particular route, the impact of cytokines on depressive states may stem from inhibition of glucocorticoid receptor function.4,232
There has been particular interest in the role of nuclear factor-κB in mediating the relation between cytokine activation and the emergence of MDD. Ordinarily, nuclear factor-κB is fundamental in mobilizing inflammatory chemokines and lymphocyte-proliferative responses to sites of infection and traumatic injury, and it has been suggested that it may be involved in promoting neuronal survival after injury to the hippocampus.74,233 The neuroprotective action of nuclear factor-κB may stem from the induction of antiapoptotic proteins such as bcl-2 and the antioxidant enzyme manganese-superoxide dismutase. Yet, nuclear factor-κB signalling may also provoke the synthesis of inflammatory cytokines, reactive oxygen species and excitotoxins that contribute to neurodegeneration.234 This might be a down-stream signalling factor for cytokines that contribute to pathologies such as Parkinson disease and multiple sclerosis,234,235 and it may be involved in MDD. Some of these changes, including nuclear factor-κB, may be instrumental in promoting depressive-like symptoms215,232,236 and may also contribute to anxiety, given that anxiety is diminished in mice deficient for the p50 subunit of nuclear factor-κB.233
Finally, cytokines might influence depressive pathology by affecting processes such as neurogenesis that are aligned with neuroplasticity.19 It has been shown that chronic interferon-α administration results in diminished hippocampal neurogenesis, and this outcome is attenuated by the interleukin-1 antagonist.237 Just as antidepressants prevent the effects of chronic stressors on neurogenesis,219 attenuation of inflammatory responses through indomethacin restore neurogenesis that was otherwise reduced by endotoxin therapy.238 Likewise, overexpression of interleukin-6 or administration of lipopolysaccharide inhibit hippocampal neurogenesis, and anti-inflammatory treatments prevent this outcome.238,239
Summary
Activation of the inflammatory immune system has been implicated as a factor that could promote MDD, presumably by acting on neurochemical processes and growth factors that are affected by traditional stressors (e.g, monoamines, CRH, BDNF). It is thought that proinflammatory cytokines entering the brain from peripheral circulation and de novo synthesis within the central nervous system may be fundamental in provoking these biological alterations. The actions of cytokines on behavioural and neurochemical processes are particularly marked when applied on a stressor backdrop, and previous stressor or cytokine elevations may augment responses to later cytokine (or stressor) challenges (sensitization). Thus current or past stressful life experiences may influence the neurochemical actions of inflammatory immune challenges and may increase vulnerability to MDD. It has been suggested that the comorbidity between MDD and several other illnesses (e.g., cardiovascular illness, diabetes, multiple sclerosis, Parkinson disease, Alzheimer disease) may involve inflammatory factors as a common denominator, and these processes may be involved in the evolution of disorders.4 It is still uncertain whether targeting inflammatory processes in the treatment of depressive disorders (or comorbid conditions) will be fruitful.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and by the Natural Sciences and Engineering Research Council of Canada. Dr. Anisman holds a Canada Research Chair in Behavioural Neuroscience.
Footnotes
Competing interests: None declared.
Correspondence to: Prof. H. Anisman, Institute of Neuroscience, Carleton University, Life Science Research Centre, Ottawa ON K1S 5B6; fax 613 520-4052; hanisman@ccs.carleton.ca
References
- 1.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 1998;105:83-107. [DOI] [PubMed]
- 2.Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 2006;110:135-370. [DOI] [PubMed]
- 3.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron 2002;34:13-25. [DOI] [PubMed]
- 4.Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol 2008;85:1-74. [DOI] [PubMed]
- 5.Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1996;1:336-42. [PubMed]
- 6.Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2002;2:23-33. [DOI] [PubMed]
- 7.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006;59:1116-27. [DOI] [PubMed]
- 8.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med 2001;7:541-7. [DOI] [PubMed]
- 9.Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol 1999;461:199-233. [DOI] [PubMed]
- 10.Dantzer R, O'Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46-56. [DOI] [PMC free article] [PubMed]
- 11.Blalock JE. The immune system as a sensory organ. J Immunol 1984;132:1067-70. [PubMed]
- 12.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 1995;19:11-38. [DOI] [PubMed]
- 13.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol 1999;461:25-45. [DOI] [PubMed]
- 14.Hayley S, Borowski T, Merali Z, et al. Central monoamine activity in genetically distinct strains of mice following a psychogenic stressor: effects of predator exposure. Brain Res 2001a;892:293-300. [DOI] [PubMed]
- 15.Merali Z, Khan S, Michaud DS, et al. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur J Neurosci 2004;20:229-39. [DOI] [PubMed]
- 16.Anisman H, Matheson K. Stress, anhedonia and depression: caveats concerning animal models. Neurosci Biobehav Rev 2005;29:525-46. [DOI] [PubMed]
- 17.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 2004;130:355-91. [DOI] [PubMed]
- 18.Anisman H, Zacharko RM. Multiple neurochemical and behavioral consequences of stressors. Implications for depression. In: File SE, editor. Psychopharmacology of anxiolytics and antidepressants. New York: Pergamon Press; 2001. p. 57-82.
- 19.Hayley S, Poulter M, Merali Z, et al. The pathogenesis of clinical depression: stressor-and cytokine-induced alterations of neuroplasticity. Neuroscience 2005;135:659-78. [DOI] [PubMed]
- 20.Stanford SC. Central noradrenergic neurones and stress. Pharmacol Ther 1995;68:297-342. [DOI] [PubMed]
- 21.Keeney A, Jessop DS, Harbuz MS, et al. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol 2006;18:330-8. [DOI] [PubMed]
- 22.Beitia G, Garmendia L, Azpiroz A, et al. Time-dependent behavioral, neurochemical, and immune consequences of repeated experiences of social defeat stress in male mice and the ameliorative effects of fluoxetine. Brain Behav Immun 2005;19:530-9. [DOI] [PubMed]
- 23.Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine responmse to stress: an electrochemical study in freely-behaving rats. Brain Res 1996;715:86-97. [DOI] [PubMed]
- 24.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 1996;721:140-9. [DOI] [PubMed]
- 25.Briones-Aranda A, Rocha L, Picazo O. Influence of forced swimming stress on 5-HT1A receptors and serotonin levels in mouse brain. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:275-81. [DOI] [PubMed]
- 26.Neumaier JF, Petty F, Kramer GL, et al. Learned helplessness increases 5-hydroxytryptamine1B receptor mRNA levels in the rat dorsal raphe nucleus. Biol Psychiatry 1997;41:668-74. [DOI] [PubMed]
- 27.Weiss JM, Goodman PA, Losito BG, et al. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine and serotonin levels in various regions of rat brain. Brain Res Rev 1981;3:167-205.
- 28.Amat J, Baratta MV, Paul E, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 2005;8:365-71. [DOI] [PubMed]
- 29.Amat J, Paul E, Zarza C, et al. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci 2006;26:13264-72. [DOI] [PMC free article] [PubMed]
- 30.Bland ST, Hargrave D, Pepin JL, et al. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology 2003;28:1589-96. [DOI] [PubMed]
- 31.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007;87:873-904. [DOI] [PubMed]
- 32.Gresch PJ, Sved AF, Zigmond MJ, et al. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem 1994;63:575-83. [DOI] [PubMed]
- 33.Buwalda B, Kole MH, Veenema AH, et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 2005;29:83-97. [DOI] [PubMed]
- 34.Stone EA. Central cyclic-AMP-linked noradrenergic receptors: new findings on properties as related to the actions of stress. Neurosci Biobehav Rev 1987;11:391-8. [DOI] [PubMed]
- 35.Bolanos-Jimenez F, Manhaes de Casatro R, Seguin L, et al. Effects of stress on the functional properties of pre-and postsynaptic 5-HT1B receptors in the brain. Eur J Pharmacol 1995;294:531-40. [DOI] [PubMed]
- 36.Dwivedi Y, Mondal AC, Payappagoudar GV, et al. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology 2005;48:204-14. [DOI] [PubMed]
- 37.Ossowska G, Nowa G, Kata R, et al. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm 2001; 108:311-9. [DOI] [PubMed]
- 38.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000;21:55-89. [DOI] [PubMed]
- 39.Lightman SL. How does the hypothalamus respond to stress? Semin Neurosci 1994;6:215-9.
- 40.Makino S, Shibasaki T, Yamauchi N, et al. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res 1999;850:136-43. [DOI] [PubMed]
- 41.Merali Z, McIntosh J, Kent P, et al. Aversive as well as appetitive events evoke the release of corticotropin releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 1998;18:4758-66. [DOI] [PMC free article] [PubMed]
- 42.Schulkin J. Angst and the amygdala. Dialogues Clin Neurosci 2006; 8: 407-16. [DOI] [PMC free article] [PubMed]
- 43.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci 2005;28:629-35. [DOI] [PubMed]
- 44.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992;15:353-75. [DOI] [PubMed]
- 45.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000;23:155-84. [DOI] [PubMed]
- 46.Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154, 526, a selective CRF1 receptor antagonist. Eur J Pharmacol 1997;323:21-6. [DOI] [PubMed]
- 47.Keck ME, Muller MB. Mutagenesis and knockout models: hypothalamic-pituitary-adrenocortical system. Handbook of Exp Pharmacol 2005;169:113-141. [DOI] [PubMed]
- 48.van Gaalen MM, Reul JM, Gesing A, et al. Mice overexpressing CRH show reduced responsiveness in plasma corticosterone after a 5-HT1A receptor challenge. Genes Brain Behav 2002;1:174-7. [DOI] [PubMed]
- 49.Iredale PA, Terwilliger R, Widnell KL, et al. Differential regulation of corticotropin-releasing factor1 receptor expression by stress and agonist treatments in brain and cultured cells. Mol Pharmacol 1996;50:1103-10. [PubMed]
- 50.Contarino A, Dellu F, Koob GF, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res 1999;835:1-9. [DOI] [PubMed]
- 51.Smith GW, Aubry JM, Dellu F, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998;20:1093-102. [DOI] [PubMed]
- 52.Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 1998;19:162-6. [DOI] [PubMed]
- 53.Keck ME, Welt T, Wigger A, et al. The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci 2001;13:373-80. [DOI] [PubMed]
- 54.Muller MB, Zimmermann S, Sillaber I, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 2003;6:1100-7. [DOI] [PubMed]
- 55.Bale TL, Contarino AB, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 2000;24:410-4. [DOI] [PubMed]
- 56.Bale TL, Vale WW. Increased depression like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. Neuroscience 2003;23:5295-301. [DOI] [PMC free article] [PubMed]
- 57.Preil J, Müller MB, Gesing A, et al. Regulation of the hypothalamic– pituitary–adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology 2001;142:4946-55. [DOI] [PubMed]
- 58.Kozlovsky N, Matar MA, Kaplan Z, et al. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int J Neuropsychopharmacol 2007;10:741-58. [DOI] [PubMed]
- 59.Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology 2002;27:133-42. [DOI] [PubMed]
- 60.Smith MA, Makino S, Kvetnansky R, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 1995;15:1768-77. [DOI] [PMC free article] [PubMed]
- 61.Yee BK, Zhu SW, Mohammed AH, et al. Levels of neurotrophic factors in the hippocampus and amygdala correlate with anxiety-and fear-related behaviour in C57BL6 mice. J Neural Transm 2007;114:431-44. [DOI] [PubMed]
- 62.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci 1999;20:59-61. [DOI] [PubMed]
- 63.Shirayama Y, Chen AC, Nakagawa S, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002;22:3251-61. [DOI] [PMC free article] [PubMed]
- 64.Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol 2007;7:18-21. [DOI] [PubMed]
- 65.Rogóz Z, Skuza G, Legutko B. Repeated co-treatment with imipramine and amantadine induces hippocampal brain-derived neurotrophic factor gene expression in rats. J Physiol Pharmacol 2007;58:219-34. [PubMed]
- 66.Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrκB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 2003;23:349-57. [DOI] [PMC free article] [PubMed]
- 67.Monteggia LM, Luikart B, Barrot M, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 2007;61:187-97. [DOI] [PubMed]
- 68.Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol Psychiatry 2003;54:59-69. [DOI] [PubMed]
- 69.Dwivedi Y, Rizavi HS, Conley RR, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003;60:804-15. [DOI] [PubMed]
- 70.Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 2003;54:70-5. [DOI] [PubMed]
- 71.Piccinni A, Marazziti D, Catena M, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord 2008;105:279-83. [DOI] [PubMed]
- 72.Huang TL, Lee CT, Liu YL. Serum brain-derived neurotrophic factor levels in patients with major depression: effects of antidepressants. J Psychiatr Res 2008;42:521-5. [DOI] [PubMed]
- 73.Rothwell NJ. Cytokines — Killers in the brain? J Physiol 1999;514:3-17. [DOI] [PMC free article] [PubMed]
- 74.Lucas PC, McAllister-Lucas LM, Nunez G. NF-kappaB signaling in lymphocytes: a new cast of characters. J Cell Sci 2004;117:31-9. [DOI] [PubMed]
- 75.Rajora N, Boccoli G, Burns D, et al. alpha-MSH modulates local and circulating tumor necrosis factor-alpha in experimental brain inflammation. J Neurosci 1997;17:2181-6. [DOI] [PMC free article] [PubMed]
- 76.Rigby WF. Drug insight: Different mechanisms of action of tumor necrosis factor antagonists-passive-aggressive behavior? Nat Clin Pract Rheumatol 2007;3:227-33. [DOI] [PubMed]
- 77.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998;281:1305-8. [DOI] [PubMed]
- 78.Dopp JM, Sarafian TA, Spinella FM, et al. Expression of the p75 TNF receptor is linked to TNF-induced NFkappaB translocation and oxyradical neutralization in glial cells. Neurochem Res 2002;27: 1535-42. [DOI] [PubMed]
- 79.Ringheim GE, Conant K. Neurodegenerative disease and the neuroimmune axis (Alzheimer's and Parkinson's disease, and viral infections). J Neuroimmunol 2004;147:43-9. [DOI] [PubMed]
- 80.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 2007;178:2623-9. [DOI] [PubMed]
- 81.Maher FO, Clarke RM, Kelly A, et al. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem 2006;96:1560-71. [DOI] [PubMed]
- 82.Pokrovskaja K, Panaretakis T, Grandér D. Alternative signaling pathways regulating type I interferon-induced apoptosis. J Interferon Cytokine Res 2005;25:799-810. [DOI] [PubMed]
- 83.Banks WA. Physiology and pathology of the blood–brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J Neurovirol 1999;5:538-55. [DOI] [PubMed]
- 84.Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, et al. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol Behav 2005;85:500-11. [DOI] [PMC free article] [PubMed]
- 85.Saija A, Princi P, Lanza M, et al. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci 1995;56:775-84. [DOI] [PubMed]
- 86.Stamatovic SM, Dimitrijevic OB, Keep RF, et al. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir Suppl 2006;96:444-50. [DOI] [PubMed]
- 87.Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res 2001;888:117-27. [DOI] [PubMed]
- 88.Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 2001;26:761-88. [DOI] [PubMed]
- 89.Rivest S, Lacroix S, Vallieres L, et al. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 2000;223:22-38. [DOI] [PubMed]
- 90.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 2002;25:154-9. [DOI] [PubMed]
- 91.Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood–brain barrier. Neuroscience 1999;93:1449-64. [DOI] [PubMed]
- 92.Kamm K, Vanderkolk W, Lawrence C, et al. The effect of traumatic brain injury upon the concentration and expression of interleukin-1beta and interleukin-10 in the rat. J Trauma 2006;60:152-7. [DOI] [PubMed]
- 93.Ledeboer A, Binnekade R, Brave JJ, et al. Site-specific modulation of LPS-induced fever and interleukin-1 beta expression in rats by interleukin-10. Am J Physiol Regul Integr Comp Physiol 2002;282: R1762-72. [DOI] [PubMed]
- 94.Zhu Y, Saito K, Murakami Y, et al. Early increase in mRNA levels of pro-inflammatory cytokines and their interactions in the mouse hippocampus after transient global ischemia. Neurosci Lett 2006;393:122-6. [DOI] [PubMed]
- 95.Sriram K, Matheson JM, Benkovic SA, et al. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-α. FASEB J 2006;20:670-82. [DOI] [PubMed]
- 96.Miyahara S, Komori T, Fujiwara R, et al. Effects of repeated stress on expression of interleukin-6 (IL-6) and IL-6 receptor mRNAs in rat hypothalamus and midbrain. Life Sci 2000;66:PL93-8. [DOI] [PubMed]
- 97.Nguyen KT, Deak T, Owens SM, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci 1998;18: 2739-46. [DOI] [PMC free article] [PubMed]
- 98.Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci 2000;917:608-17. [DOI] [PubMed]
- 99.Tilders FJH, Schmidt ED. Cross-sensitization between immune and non-immune stressors. A role in the etiology of depression? Adv Exp Med Biol 1999;461:179-97. [DOI] [PubMed]
- 100.Hayley S, Staines WM, Merali Z, et al. Time-dependent sensitization of corticotropin-releasing hormone, arginine vasopressin and c-fos immunoreactivity within the mouse brain in response to tumor necrosis factor-alpha. Neuroscience 2001;106:137-48. [DOI] [PubMed]
- 101.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997;20:78-84. [DOI] [PubMed]
- 102.Day HE, Curran EJ, Watson SJ. Jr, et al. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 1999;413:113-28. [PubMed]
- 103.Hayley S, Brebner K, Lacosta S, et al. Sensitization to the effects of tumor necrosis factor-a; neuroendocrine, central monoamine and behavioral variations. J Neurosci 1999;19:5654-65. [DOI] [PMC free article] [PubMed]
- 104.Linthorst ACE, Flachskamm C, Muller-Preuss P, et al. Effect of bacterial endotoxin and interleukin-1β on hippocampal serotonergic neurotransmision, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci 1995;15:2920-34. [DOI] [PMC free article] [PubMed]
- 105.Merali Z, Lacosta S, Anisman H. Effects of interleukin-1β and mild stress on alterations of central monoamines: a regional microdialysis study. Brain Res 1997;761:225-35. [DOI] [PubMed]
- 106.Shintani F, Kanba S, Nakaki T, et al. Interleukin-1β augments release of norepinephrine, dopamine and serotonin in the rat anterior hypothalamus. J Neurosci 1993;13:3574-81. [DOI] [PMC free article] [PubMed]
- 107.Shintani F, Nakaki T, Kanba S, et al. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci 1995;15:1961-70. [DOI] [PMC free article] [PubMed]
- 108.Song C, Merali Z, Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 1999;88:823-36. [DOI] [PubMed]
- 109.Zhang J, Terreni L, De Simoni MG, et al. Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochem Int 2001;38:303-8. [DOI] [PubMed]
- 110.Zalcman S, Green-Johnson JM, Murray L, et al. Cytokine-specific central monoamine alterations induced by interleukin (IL)-1, IL-2 and IL-6. Brain Res 1994;643:40-9. [DOI] [PubMed]
- 111.Bluthe RM, Pawlowski M, Suarez S, et al. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 1994;19:197-207. [DOI] [PubMed]
- 112.Brebner K, Hayley S, Merali Z, et al. Synergistic effects of interleukin-1, interleukin-6, and TNF-α: neuroendocrine, neurochemical and behavioral changes. Neuropsychopharmacology 2000;22:566-80. [DOI] [PubMed]
- 113.van der Meer MJ, Lebeau A, Duval P, et al. Synergism between IL-1 beta and TNF-alpha on the activity of the pituitary-adrenal axis and on food intake of rats. Am J Physiol 1995;268:E551-7. [DOI] [PubMed]
- 114.Zhou D, Shanks N, Riechman SE, et al. Interleukin 6 modulates interleukin-1 and stress-induced activation of the hypothalamic– pituitary–adrenal axis in male rats. Neuroendocrinology 1996;63:227-36. [DOI] [PubMed]
- 115.Perlstein RS, Mougey EH, Jackson WE, et al. Interleukin-1 and interleukin-6 act synergistically to stimulate the release of adrenocorticotropic hormone in vivo. Lymphokine Cytokine Res 1991;10:141-6. [PubMed]
- 116.Anisman H, Poulter MO, Gandhi R, et al. Interferon-alpha effects are exaggerated when administered on a psychosocial stressor backdrop: cytokine, corticosterone and brain monoamine variations. J Neuroimmunol 2007;186:45-53. [DOI] [PubMed]
- 117.Gandhi R, Hayley S, Gibb J, et al. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav Immun 2007;21:477-89. [DOI] [PubMed]
- 118.Gibb J, Hayley S, Gandhi R, et al. Synergistic actions of psychosocial and endotoxin challenges: circulation cytokine, corticosterone and behavioral changes. Brain Behav Immun 2008;22:573-89. [DOI] [PubMed]
- 119.Goshen I, Kreisel T, Ben-Menachem-Zidon O, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. In press. [DOI] [PubMed]
- 120.Rage F, Silhol M, Tapia-Arancibia L. IL-1beta regulation of BDNF expression in rat cultured hypothalamic neurons depends on the presence of glial cells. Neurochem Int 2006;49:433-41. [DOI] [PubMed]
- 121.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol 2006;1:212-22. [DOI] [PMC free article] [PubMed]
- 122.Schulte-Herbrüggen O, Nassenstein C, Lommatzsch M, et al. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol 2005;160:204-9. [DOI] [PubMed]
- 123.Churchill L, Taishi P, Wang M, et al. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res 2006;1120:64-73. [DOI] [PubMed]
- 124.Tong L, Balazs R, Soiampornkul R, et al. Interleukin-1beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging 2007 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 125.Barrientos RM, Sprunger DB, Campeau S, et al. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol 2004;155:119-26. [DOI] [PubMed]
- 126.Hori T, Katafuchi T, Take S, et al. Neuroimmunomodulatory actions of hypothalamic interferon-alpha. Neuroimmunomodulation 1998;5:172-7. [DOI] [PubMed]
- 127.Saphier D. Neuroendocrine effects of interferon-alpha in the rat. Adv Exp Med Biol 1995;373:209-18. [DOI] [PubMed]
- 128.Raber J, Koob GF, Bloom FE. Interferon-alpha and transforming growth factor-beta 1 regulate corticotropin-releasing factor release from the amygdala: comparison with the hypothalamic response. Neurochem Int 1997;30:455-63. [DOI] [PubMed]
- 129.Schaefer M, Schwaiger M, Pich M, et al. Neurotransmitter changes by interferon-alpha and therapeutic implications. Pharmacopsychiatry 2003;36:S203-6. [DOI] [PubMed]
- 130.Yokoyama M, Suzuki E, Sato T, et al. Effects of intraperitoneal administration of IFN-alpha for one, four, and fourteen days on amino acid levels in various rat brain regions. J Interferon Cytokine Res 2005;25:187-91. [DOI] [PubMed]
- 131.Morikawa O, Sakai N, Obara H, et al. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol 1998;349:317-24. [DOI] [PubMed]
- 132.Kitagami T, Yamada K, Miura H, et al. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood–brain barrier. Brain Res 2003;978:104-14. [DOI] [PubMed]
- 133.Shuto H, Kataoka Y, Horikawa T, et al. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res 1997;747:348-51. [DOI] [PubMed]
- 134.Asnis GM, De la Garza R II, Kohn SR, et al. IFN-induced depression: a role for NSAIDs. Psychopharmacol Bull 2003;37:29-50. [PubMed]
- 135.De La Garza R, Asnis GM, Pedrosa E, et al. Recombinant human interferon-alpha does not alter reward behavior, or neuroimmune and neuroendocrine activation in rats. Prog Neuropsychopharmacol Biol Psychiatry 2005;9:781-92. [DOI] [PubMed]
- 136.Yang W, Wang Q, Kanes SJ, et al. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res Mol Brain Res 2004;124:70-8. [DOI] [PubMed]
- 137.Merali Z, Bédard T, Andrews N, et al. Bombesin receptors as novel anti-anxiety therapeutic target; non-peptide antagonist PD 176252 reduces anxiety and 5-HT release through BB1 receptor. J Neurosci 2006;26:10387-96. [DOI] [PMC free article] [PubMed]
- 138.Gurevich I, Englander MT, Adlersberg M, et al. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci 2002;22:10529-32. [DOI] [PMC free article] [PubMed]
- 139.Cassidy EM, Manning D, Byrne S, et al. Acute effects of low-dose interferon-alpha on serum cortisol and plasma interleukin-6. J Psychopharmacol 2002;16:230-4. [DOI] [PubMed]
- 140.Shimizu H, Ohtani K, Sato N, et al. Increase in serum interleukin-6, plasma ACTH and serum cortisol levels after systemic interferon-alpha administration. Endocr J 1995;42:551-6. [DOI] [PubMed]
- 141.Gisslinger H, Svoboda T, Clodi M, et al. Interferon-alpha stimulates the hypothalamic–pituitary–adrenal axis in vivo and in vitro. Neuroendocrinology 1993;57:489-95. [DOI] [PubMed]
- 142.Anisman H, Hayley S, Merali Z. Cytokines and stress: sensitization and cross-sensitization. Brain Behav Immun 2003;17:86-93. [DOI] [PubMed]
- 143.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010. [DOI] [PubMed]
- 144.Kendler KS, Thornton LM, Gardner CO. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. Am J Psychiatry 2001;158:582-6. [DOI] [PubMed]
- 145.Wedzony K, Mackowiak M, Fijal K, et al. Evidence that conditioned stress enhances outflow of dopamine in rat prefrontal cortex: a search for the influence of diazepam and 5-HT1A agonists. Synapse 1996;24:240-7. [DOI] [PubMed]
- 146.Harvey BH, Naciti C, Brand L, et al. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res 2003;983:97-107. [DOI] [PubMed]
- 147.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 2005;29:829-41. [DOI] [PubMed]
- 148.Pacchioni AM, Gioino G, Assis A, et al. A single exposure to restraint stress induces behavioral and neurochemical sensitization to stimulating effects of amphetamine: involvement of NMDA receptors. Ann N Y Acad Sci 2002;965:233-46. [DOI] [PubMed]
- 149.Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berl) 2000;151:152-65. [DOI] [PubMed]
- 150.Hayley S, Wall P, Anisman H. Sensitization to the neuroendocrine, central monoamine and behavioral effects of murine tumor necrosis factor-α: peripheral and central mechanisms. Eur J Neurosci 2002;15:1061-76. [DOI] [PubMed]
- 151.Tilders FJH, Schmidt ED, De Goeij DCE. Phenotypic plasticity of CRF neurons during stress. Ann N Y Acad Sci 1993;697:39-52. [DOI] [PubMed]
- 152.Schmidt ED, Janszen AWJW, Wouterlood FG, et al. Interleukin-1 induced long-lasting changes in hypothalamic corticotropin-releasing hormone (CRH) neurons and hyperresponsiveness of the hypothalamic–pituitary–adrenal axis. J Neurosci 1995;15:7417-26. [DOI] [PMC free article] [PubMed]
- 153.Johnson JD, O'Connor KA, Watkins LR, et al. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience 2004;127:569-77. [DOI] [PubMed]
- 154.Bonaccorso S, Maier SF, Meltzer HY, et al. Behavioral changes in rats after acute, chronic and repeated administration of interleukin-1beta: relevance for affective disorders. J Affect Disord 2003;77:143-8. [DOI] [PubMed]
- 155.Maes M, Ombelet W, De Jongh R, et al. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord 2001;63:85-92. [DOI] [PubMed]
- 156.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic–pituitary–adrenal function and health. Trends Mol Med 2007l;13:269-77. [DOI] [PubMed]
- 157.Shanks N, Windle RJ, Perks PA, et al. Early-life exposure to endotoxin alters hypothalamic–pituitary–adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A 2000;97:5645-50. [DOI] [PMC free article] [PubMed]
- 158.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci 2001;933:222-34. [DOI] [PubMed]
- 159.Kusnecov AW, Liang R, Shurin G. T-lymphocyte activation increases hypothalamic and amygdaloid expression of CRH mRNA and emotional reactivity to novelty. J Neurosci 1999;19:4533-43. [DOI] [PMC free article] [PubMed]
- 160.Bret-Dibat JL, Bluthe RM, Kent S, et al. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun 1995;9:242-6. [DOI] [PubMed]
- 161.Bluthe RM, Dantzer R, Kelley KW. Central mediation of the effects of interleukin-1 on social explorationand body weight in mice. Psychoneuroendocrinology 1997;22:1-11. [DOI] [PubMed]
- 162.Yirmiya R, Pollak Y, Barak O, et al. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology 2001;24:531-44. [DOI] [PubMed]
- 163.Castanon N, Konsman JP, Medina C, et al. Chronic treatment with the antidepressant tianeptine attenuates lipopolysaccharide-induced Fos expression in the rat paraventricular nucleus and HPA axis activation. Psychoneuroendocrinology 2003;28:19-34. [DOI] [PubMed]
- 164.De La Garza R, Fabrizio KR, Radoi GE, et al. Diclofenac sodium attenuates lipopolysaccharide-induced alterations to reward behavior and corticosterone release. Behav Brain Res 2004;149:77-85. [DOI] [PubMed]
- 165.Bugajski J, Gadek-Michalska A, Bugajski AJ. Effect of cyclo-oxygenase inhibitors on the CRH-induced pituitary-adrenocortical activity during crowding stress. J Physiol Pharmacol 2003;54:99-108. [PubMed]
- 166.Hollis JH, Evans AK, Bruce KP, et al. Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain Behav Immun 2006;20:569-77. [DOI] [PubMed]
- 167.Anisman H, Kokkinidis L, Borowski T, et al. Differential effects of IL-1, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res 1998;779:177-87. [DOI] [PubMed]
- 168.Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun 2002;16:544-56. [DOI] [PubMed]
- 169.Merali Z, Brennan K, Brau P, et al. Dissociating anorexia and anhedonia elicited by interleukin-1β: antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology (Berl) 2002;165:413-8. [DOI] [PubMed]
- 170.Larson SJ, Romanoff RL, Dunn AJ, et al. Effects of interleukin-1beta on food-maintained behavior in the mouse. Brain Behav Immun 2002;16:398-410. [DOI] [PubMed]
- 171.Borowski T, Kokkinidis L, Merali Z, et al. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport 1998;9:3797-802. [DOI] [PubMed]
- 172.van der Meer MJ, Sweep CG, Pesman GJ, et al. Chronic stimulation of the hypothalamus-pituitary-adrenal axis in rats by interleukin 1beta: central and peripheral mechanisms. Cytokine 1996;8:910-9. [DOI] [PubMed]
- 173.Parsadaniantz SM, Lebeau A, Duval P, et al. Effects of the inhibition of cyclo-oxygenase 1 or 2 or 5-lipoxygenase on the activation of the hypothalamic-pituitary-adrenal axis induced by interleukin-1beta in the male rat. J Neuroendocrinol 2000;12:766-73. [DOI] [PubMed]
- 174.Niimi M, Sato M, Wada Y, et al. Effect of continuous intravenous injection of interleukin-6 and pretreatment with cyclooxygenase inhibitor on brain c-fos expression in the rat. Neuroendocrinology 1997;66:47-53. [DOI] [PubMed]
- 175.Fahey B, Hickey B, Kelleher D, et al. The widely-used anti-viral drug interferon-alpha induces depressive-and anxiogenic-like effects in healthy rats. Behav Brain Res 2007;182:80-7. [DOI] [PubMed]
- 176.Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 2001;15:199-226. [DOI] [PubMed]
- 177.Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol 2008;18:230-3. [DOI] [PMC free article] [PubMed]
- 178.Basterzi AD, Aydemir C, Kisa C, et al. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum Psychopharmacol 2005;20:473-6. [DOI] [PubMed]
- 179.Sluzewska A, Rybakowski JK, Laciak M, et al. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci 1995;762:474-6. [DOI] [PubMed]
- 180.Tuglu C, Kara SH, Caliyurt O, et al. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003;170:429-33. [DOI] [PubMed]
- 181.Anisman H, Ravindran AV, Griffiths J, et al. Interleukin-1 beta production in dysthymia before and after pharmacotherapy. Biol Psychiatry 1999b;46:1649-55. [DOI] [PubMed]
- 182.Eller T, Vasar V, Shlik J, et al. Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:445-50. [DOI] [PubMed]
- 183.Irwin M. Immune correlates of depression. Adv Exp Med Biol 1999;461:1-24. [DOI] [PubMed]
- 184.Haack M, Hinze-Selch D, Fenzel T, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res 1999;33:407-18. [DOI] [PubMed]
- 185.Cover H, Irwin M. Immunity and depression: insomnia, retardation and reduction of natural killer cell activity. J Behav Med 1994;17: 217-23. [DOI] [PubMed]
- 186.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001;58:445-52. [DOI] [PubMed]
- 187.Wright CE, Strike PC, Brydon L, et al. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun 2005;19:345-50. [DOI] [PubMed]
- 188.Bonaccorso S, Puzella A, Marino V, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol 2002;22:86-90. [DOI] [PubMed]
- 189.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol 2000;18:2143-51. [DOI] [PubMed]
- 190.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002;26:643-52. [DOI] [PubMed]
- 191.Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry 2003;160:1342-5. [DOI] [PubMed]
- 192.Maes M, Bonaccorso S. Lower activities of serum peptidases predict higher depressive and anxiety levels following interferon-alpha-based immunotherapy in patients with hepatitis C. ACTA Psychiat Scand 2004;109:126-131. [DOI] [PubMed]
- 193.Maes M, Capuron L, Ravaud A, et al. Lowered serum dipeptidyl peptidase IV activity is associated with depressive symptoms and cytokine production in cancer patients receiving interleukin-2-based immunotherapy. Neuropsychopharmacology 2001;24:130-40. [DOI] [PubMed]
- 194.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of the depression and neurotoxicity induced by high dose interferon alpha. N Engl J Med 2001;344:961-6. [DOI] [PubMed]
- 195.Scalori A, Pozzi M, Bellia V, et al. Interferon-induced depression: prevalence and management. Dig Liver Dis 2005;37:102-7. [DOI] [PubMed]
- 196.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci 2004;29:11-7. [PMC free article] [PubMed]
- 197.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol 2002;5:375-88. [DOI] [PubMed]
- 198.Valentine AD, Meyers CA, Talpaz M. Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest 1995;13:561-6. [DOI] [PubMed]
- 199.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord 2004;82:175-90. [DOI] [PubMed]
- 200.Quarantini LC, Bressan RA, Galvão A, et al. Incidence of psychiatric side effects during pegylated interferon-alpha retreatment in nonresponder hepatitis C virus-infected patients. Liver Int 2007;27: 1098-102. [DOI] [PubMed]
- 201.Beratis S, Katrivanou A, Georgiou S, et al. Major depression and risk of depressive symptomatology associated with short-term and low-dose interferon-alpha treatment. J Psychosom Res 2005;58:15-8. [DOI] [PubMed]
- 202.Capuron L, Ravaud A, Miller AH, et al. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun 2004;18:205-13. [DOI] [PubMed]
- 203.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav 2002;71:857-65. [DOI] [PubMed]
- 204.Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry 2002;7:468-73. [DOI] [PubMed]
- 205.Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72-week prospective study. AIDS 2005;19 Suppl 3:S174-8. [DOI] [PubMed]
- 206.Wichers MC, Kenis G, Leue C, et al. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry 2006;60:77-9. [DOI] [PubMed]
- 207.Robaeys G, De Bie J, Wichers MC, et al. Early prediction of major depression in chronic hepatitis C patients during peg-interferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World J Gastroenterol 2007;13:5736-40. [DOI] [PMC free article] [PubMed]
- 208.Kraus MR, Schafer A, Al-Taie O, et al. Prophylactic SSRI during interferon alpha re-therapy in patients with chronic hepatitis C and a history of interferon-induced depression. J Viral Hepat 2005;12:96-100. [DOI] [PubMed]
- 209.Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther 2002;16:1091-9. [DOI] [PubMed]
- 210.Maddock C, Baita A, Orru MG, et al. Psychopharmacological treatment of depression, anxiety, irritability and insomnia in patients receiving interferon-alpha: a prospective case series and a discussion of biological mechanisms. J Psychopharmacol 2004;18:41-6. [DOI] [PubMed]
- 211.Schramm TM, Lawford BR, Macdonald GA, et al. Sertraline treatment of interferon-alfa-induced depressive disorder. Med J Aust 2000;173:359-61. [DOI] [PubMed]
- 212.Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther 2007;25:1163-74. [DOI] [PubMed]
- 213.Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol 2005;42:793-8. [DOI] [PubMed]
- 214.Morasco BJ, Rifai MA, Loftis JM, et al. A randomized trial of paroxetine to prevent interferon-alpha-induced depression in patients with hepatitis C. J Affect Disord 2007;103:83-90. [DOI] [PubMed]
- 215.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27:24-31. [DOI] [PMC free article] [PubMed]
- 216.Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 2005;19:105-23. [DOI] [PMC free article] [PubMed]
- 217.Muller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 2006;11:680-4. [DOI] [PubMed]
- 218.de Jong JC, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol 2003;55:591-5. [DOI] [PMC free article] [PubMed]
- 219.Chiou SH, Chen SJ, Peng CH, et al. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun 2006;343:391-400. [DOI] [PubMed]
- 220.Reynolds JL, Ignatowski TA, Sud R, et al. An antidepressant mechanism of desipramine is to decrease tumor necrosis factor-alpha production culminating in increases in noradrenergic neurotransmission. Neuroscience 2005;133:519-31. [DOI] [PubMed]
- 221.Reynolds JL, Ignatowski TA, Sud R, et al. Brain derived tumor necrosis factor-alpha and its involvement in noradrenergic neuron functioning involved in the mechanism of action of an antidepressant. J Pharmacol Exp Ther 2004;310:1216-25. [DOI] [PubMed]
- 222.Myint AM, O'Mahony S, Kubera M, et al. Role of paroxetine in interferon-alpha-induced immune and behavioural changes in male Wistar rats. J Psychopharmacol 2007;21:843-50. [DOI] [PubMed]
- 223.Brustolim D, Ribeiro-dos-Santos R, Kast RE, et al. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 2006;6:903-7. [DOI] [PubMed]
- 224.Obuchowicz E, Kowalski J, Labuzek K, et al. Amitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell cultures. Int J Neuropsychopharmacol 2006;9:27-35. [DOI] [PubMed]
- 225.Griswold DE, Webb EF, Badger AM, et al. SB 207499 (Ariflo), a second generation phosphodiesterase 4 inhibitor, reduces tumor necrosis factor alpha and interleukin-4 production in vivo. J Pharmacol Exp Ther 1998;287:705-11. [PubMed]
- 226.Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev 2001;7:387-98. [DOI] [PMC free article] [PubMed]
- 227.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry 2005;66:1050-7. [DOI] [PubMed]
- 228.Dieperink E, Ho SB, Thuras P, et al. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics 2003;44:104-12. [DOI] [PubMed]
- 229.Lieb K, Engelbrecht MA, Gut O, et al. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNalpha): results from a prospective study. Eur Psychiatry 2006;21:204-10. [DOI] [PubMed]
- 230.Charlton BG. The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Med Hypotheses 2000;54:126-30. [DOI] [PubMed]
- 231.Wichers MC, Koek GH, Robaeys G, et al. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 2005;10:538-44. [DOI] [PubMed]
- 232.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 2007;21:9-19. [DOI] [PMC free article] [PubMed]
- 233.Kassed CA, Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res 2004;154:577-84. [DOI] [PubMed]
- 234.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 2004;27:589-94. [DOI] [PubMed]
- 235.Barger SW, Moerman AM, Mao X. Molecular mechanisms of cytokine-induced neuroprotection: NFkappaB and neuroplasticity. Curr Pharm Des 2005;11:985-98. [DOI] [PubMed]
- 236.Miller AH, Raison CL. Cytokines, p38 MAP kinase and the pathophysiology of depression. Neuropsychopharmacology 2006;31:2089-90. [DOI] [PubMed]
- 237.Kaneko N, Kudo K, Mabuchi T, et al. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology 2006;31:2619-26. [DOI] [PubMed]
- 238.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003;302:1760-5. [DOI] [PubMed]
- 239.Vallieres L, Campbell IL, Gage FH, et al. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 2002;22:486-92. [DOI] [PMC free article] [PubMed]