Abstract
Objective
Based on the reported association between cytokines with depression and suicide, and evidence of increased markers of inflammation in the brain of suicide victims, the present study examined the expression of cytokines in the orbitofrontal cortex of suicide victims.
Method
In a postmortem sample obtained from the Brodman area 11 of suicides (n = 34) and controls (n = 17), real-time RT-PCR was used to compare the expression of mRNA species for tumor necrosis factor-a (TNF-α), interleukin (IL)-1β, 4, 5, 6, and 13.
Results
Increased expression of IL-4 was found in women suicide victims and IL-13 in men suicide victims. Elevated but not significant cytokine expression was also observed for TNF-α in women suicide victims.
Conclusion
To our knowledge, these results provide the first evidence of the presence of mRNA transcripts of type 2 T-helper cytokines in the human orbitofrontal cortex and their increased expression in the brain of suicides.
Keywords: suicide, postmortem, gene expression, brain, neuroimmune
Significant outcomes.
Neuroimmune abnormalities in the brains of suicide completers are associated with type-2 (TH2) cytokines.
These results are consistent with the reported association between allergies and suicide.
Limitations.
Suicides and controls not matched for age.
Limited information on psychiatric diagnoses and blood toxicology in the suicide group.
Introduction
Activation of the immune system by means of cytokine therapy is known to produce psychiatric side effects. The most common consequence of administering cytokines is the triggering of symptoms of depression (1–4). In addition, other serious mental complications are the occurrence of suicidal ideation, suicidal attempts, and completion (5–8). Case reports worldwide have documented death by suicide in patients receiving cytokines as immune boosting therapy to treat a variety of diseases including melanoma, hepatitis C, HIV infections, and multiple sclerosis (5–9). While there is a growing recognition of the participation of cytokines in the etiology of mood disorders, their role as pro-suicidal factors has been less studied (10, 11).
In a recent study performed in postmortem brain tissue of patients that suffered from different psychiatric conditions including schizophrenia, major depression, and bipolar disorders, a positive correlation between markers of immune activation with suicide independent of the psychiatric disease was detected (12). This study reported that microglial cells in the frontal cortex, hippocampus, and dorsal thalamus of suicide victims had increased expression of markers of activation such as class II major histocompatible complex molecules. The regions of the brain examined have been reported to show neurochemical abnormalities in suicides and in depressed individuals and the authors suggested that some of these effects could be due to the actions of cytokines. However, data on cytokine expression in specific regions of the human brain are not yet available.
Based on the capacity of the brain to express mainly certain cytokines including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 after activation of the immune system (13–15), the first objective of the present study was to compare the expression of these cytokines in the orbitofrontal cortical Brodman area 11 of suicides and controls. This area of the human cortex was selected based on its function in the neurobiology of suicidal behavior evidenced by postmortem and functional imaging studies (16–18). Moreover, in a recent epidemiological survey, we reported the association of suicide completion with increased aeroallergens during high atmospheric concentration of pollen (19). In addition, other epidemiological studies have reported the association of allergies with depression (20–22), and possibly suicide (23). Therefore, the second objective was to compare the expression of the major cytokines involved in allergies, including IL-4, IL-5, and IL-13.
Aims of the study
The aims of the study were to compare by real-time RT-PCR the expression of mRNA species for cytokines in postmortem brain samples of victims of suicide respect to controls that died of other causes.
Material and methods
Tissue collection and autopsy of brain samples
The human brain tissue was obtained from the Institute of Forensic Medicine of the Johann Wolfgang Goethe University in Frankfurt, Main, Germany. The research was approved by the Institutional Review Board of the University. The basic characteristics of the subjects are shown in Table 1. Information on demographic data, cause of death and psychopathology of the decedents were derived from medical records, investigations of the coroner, the medical results of the examiner and interviews of next-of-kin. Postmortem tissue was derived from the orbitofrontal area (Brodmann area 11) of 34 suicides and 17 controls. Samples were selected by excluding those cases that showed signs of infarcts, subarachnoid hemorrhages or tumors. Almost all the subjects of our sample experienced brief deaths due to violent suicide methods or cardiac events with short states of agony (Table 1). All the subjects were Caucasian of German nationality.
Table 1.
Demographic and tissue characteristics of suicides and controls subjects. Mean ± standard deviation (SD) values for each group are provided for age, postmortem interval (PM h) and pH
| Group | Sex | Age | Cause of death | Diagnosis (DSM-IV) | BAL | PM h | pH |
|---|---|---|---|---|---|---|---|
| Suicide | F | 42 | Intoxication | Neg. | 11 | 6.70 | |
| Suicide | F | 49 | Hanging | MDD | 0.6 | 72 | 6.75 |
| Suicide | F | 38 | Hanging | Neg. | 66 | 6.80 | |
| Suicide | F | 61 | Hanging | 1.56 | 91 | 6.30 | |
| Suicide | F | 83 | Hanging | Neg. | 48 | 6.50 | |
| Suicide | F | 63 | Jumping from height | MDD | 52 | 6.65 | |
| Suicide | F | 39 | Jumping from height | 1.91 | 11 | 6.85 | |
| Suicide | F | 67 | Jumping from height | Neg. | 115 | 6.35 | |
| Suicide | F | 57 | Intoxication | MDD | Neg. | 110 | 6.30 |
| Suicide | F | 81 | Cutting | MDD | Neg. | 69 | 6.65 |
| Suicide | F | 31 | Intoxication | BD I | 72 | 7.00 | |
| Suicide | F | 65 | Intoxication | 48 | 6.60 | ||
| Suicide | F | 46 | Intoxication | 1.27 | 39 | 6.65 | |
| Suicide | F | 53 | Jumping from height | MDD | 0.002 | 4 | 6.75 |
| n = 14 (Mean ± SD) | 55.3 ± 15.7 | 57.7 ± 34.5 | 6.6 ± 0.2 | ||||
| Suicide | M | 33 | Gunshot | 2.24 | 24 | 6.90 | |
| Suicide | M | 59 | Hanging | MDD | 72 | 6.90 | |
| Suicide | M | 33 | Hanging | Neg. | 24 | 6.70 | |
| Suicide | M | 51 | Hanging | 0.19 | 115 | 6.80 | |
| Suicide | M | 72 | Jumping from height | Neg. | 86 | 6.85 | |
| Suicide | M | 55 | Hanging | Neg. | 88 | 6.75 | |
| Suicide | M | 57 | Hanging | 1.7 | 97 | 6.70 | |
| Suicide | M | 66 | Hanging | MDD | Neg. | 48 | 6.85 |
| Suicide | M | 72 | Hanging | 1.59 | 92 | 6.70 | |
| Suicide | M | 29 | Hanging | 0.96 | 32 | 6.60 | |
| Suicide | M | 27 | Hanging | 0.29 | 115 | 6.90 | |
| Suicide | M | 38 | Jumping from height | MDD | Neg. | 48 | 6.90 |
| Suicide | M | 60 | Hanging | AA | 0.04 | 96 | 6.70 |
| Suicide | M | 48 | Hanging | 48 | 6.60 | ||
| Suicide | M | 66 | Hanging | Neg. | 110 | 6.70 | |
| Suicide | M | 57 | Intoxication | MDD | 72 | 6.65 | |
| Suicide | M | 43 | Gunshot | AA | 2.92 | 100 | 7.10 |
| Suicide | M | 64 | Cutting | Neg. | 13 | 6.75 | |
| Suicide | M | 42 | Hanging | 0.001 | 40 | 7.05 | |
| Suicide | M | 33 | Suffocation | Neg. | 31 | 6.7 | |
| n = 20 (Mean ± SD) | 50.2 ± 14.6 | 67.5 ± 32.4 | 6.8 ± 0.1 | ||||
| Control | F | 67 | Heart attack | 72 | 6.40 | ||
| Control | F | 71 | Pancreatitis | 48 | 6.55 | ||
| Control | F | 76 | Heart attack | 115 | 6.60 | ||
| Control | F | 53 | Heart attack | 67 | 6.75 | ||
| Control | F | 62 | Heart attack | 115 | 6.80 | ||
| n = 5 (Mean ± SD) | 65.8 ± 8.8 | 83 ± 30.2 | 6.64 ± 0.1 | ||||
| Control | M | 63 | Emphysema | 98 | 6.95 | ||
| Control | M | 59 | Heart attack | 48 | 6.80 | ||
| Control | M | 65 | Heart attack | 87 | 7.00 | ||
| Control | M | 77 | Aneurysm | 76 | 6.80 | ||
| Control | M | 62 | Accident | 1.68 | 15 | 6.70 | |
| Control | M | 69 | Heart attack | 0.23 | 110 | 6.80 | |
| Control | M | 54 | Heart attack | 46 | 6.80 | ||
| Control | M | 37 | Heart attack | 61 | 6.65 | ||
| Control | M | 58 | Heart attack | 96 | 6.65 | ||
| Control | M | 48 | Heart attack | 48 | 6.95 | ||
| Control | M | 54 | Heart attack | 0.41 | 48 | 6.30 | |
| Control | M | 55 | Heart attack | 70 | 6.45 | ||
| n = 12 (Mean ± SD) | 58.4 ± 10.2 | 66.92 ± 27.6 | 6.7 ± 0.2 | ||||
| Total suicides (n = 34), mean ± SD | 52.3 ± 15 | 63.5 ± 33 | 6.7 ± 0.2 | ||||
| Total controls (n = 17) | 60.6 ± 10 | 71.7 ± 28 | 6.7 ± 0.19 |
BAL, blood alcohol level; PM h, postmortem interval; AA, alcohol abuse; BD I, bipolar I disorder; MDD, major depressive disorder.
RNA extraction and purification
Tissue pH is a robust indicator of RNA quality (24), and can affect mRNA determinations. The extraction of intact and biologically active mRNA after more than 4 days was reported by several groups (25–27). Therefore, we selected subjects with longer postmortem intervals (PMI) and focused on appropriate pH-values and stringent RNA quality control. As a result of this approach, pH values of suicides and controls were very similar (Table 1). The pH-value was measured by means of a pH-meter after homogenizing approximately 500 mg of brain tissue in 2 ml distilled water.
RNA was isolated from approximately 75 mg of frozen brain tissue using the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). The tissue samples were homogenized in 1 ml Qiazol Reagent using an Ultraturrax at 4°C for 20 s. The subsequent steps were carried out according to the manufacturers’ protocol with an additional DNAse digestion to remove potential genomic DNA. The quality of the isolated RNA was first assessed by agarose gel electophoresis, and subsequently analyzed with the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). All RNA samples showed clearly defined 28S and 18S ribosomal bands, indicating good RNA quality as proposed for quantitative PCR studies (28). The mean ribosomal RNA ratio (28S/18S), measured with the Agilent 2100 Bioanalyzer, was 1.60. The isolated RNA was stored at −80°C until further processing. Samples were shipped in dry ice to the University of Maryland within a 48 h window of time and were confirmed to be frozen at the time of arrival.
Real time RT-PCR
Five hundred nanograms of total RNA per sample were reverse transcribed into cDNA in a 20 μl reaction using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to manufacturer's instructions. This reagent contains a mixture of oligo (dT) and random hexamer primers that allow effcient reverse transcription of diverse RNA species. Real-time RT-PCR was conducted using the iQ SYBR Green Supermix (Bio-Rad) in a 50 μl reaction using the set of primers listed in Table 2. All sets of primers were tested in 1.8% agarose gel to confirm a single amplification product. The amplified products for cytokines were directly cloned into the pCRII-Topo vector (Invitrogen, Paisley, Scotland, UK) and sequenced to confirm their identity. All the primer pairs were designed using the Accelrys Gene 2.0v software (San Diego, CA, USA).
Table 2.
Primer sequences used in realtime RT-PCR determinations
| Gene | GeneBank | Primer sequence | Region | Product (bp) | |
|---|---|---|---|---|---|
| TNF-α | NM_000594 | Fwd | AAGCAACAAGACCACCACTTCG | 1010−1157 | 148 |
| Rev | TCTCCAGATTCCAGATGTCAGGG | ||||
| IL1-β | NM_000576 | Fwd | GCACCTTCTTTCCCTTCATCTTTG | 359−486 | 128 |
| Rev | GCTTTTTTGCTGTGAGTCCCG | ||||
| IL-6 | NM_000600 | Fwd | GAGAAGATTCCAAAGATGTAGCCG | 160−255 | 96 |
| Rev | AGATGCCGTCGAGGATGTACC | ||||
| IL-4 | NM_000589 | Fwd | AAGCAAAAAGCCAGCAGCAGCC | 10−94 | 85 |
| Rev | ACAAAGTTTCAGCATAGGAAATTAC | ||||
| IL-5 | NM_00879 | Fwd | GCATTGGTGAAAGAGACCTTGG | 126−187 | 62 |
| Rev | TCATTGGCTATCAGCAGAGTTCG | ||||
| IL-13 | NM_002188 | Fwd | ACCCACTTCACACACAGGCAAC | 794−918 | 124 |
| Rev | ACAGTCTTCCCCAATCCCCAAC | ||||
| 18s | K03432 | Fwd | CCGATAACGAACGAGACTCTGG | 1514−1606 | 93 |
| Rev | TGAACGCCACTTGTCCCTCTAAG | ||||
| GAPDH | NM_002046 | Fwd | TTCGTCATGGGTGTGAACC | 493−631 | 139 |
| Rev | TGGTCATGAGTCCTTCCAC | ||||
| ACTB | NM_001101 | Fwd | CCACGAAACTACCTTCAACTC | 895−1027 | 133 |
| Rev | AGTGATCTCCTTCTGCATCC |
TNF-α, tumor necrosis factor alpha; IL, interleukin; GAPDH, gliceraldehyde-3-phosphate dehydrogenase; ACTB, actin-β.
The real-time PCR reaction was run on a MyiQ instrument (Bio-Rad) with a three step cycling program as follows: an initial hot start for 5 min at 95°C followed by 40 cycles with a denaturation step of 15 s at 95°C, an annealing step of 30 s at 55°C, an extension step of 30 s at 72°C with the optics on at this last step. In preparation of a melt curve, the samples were heated for 1 min at 95°C then cooled for 1 min at 55°C, and the melt curve was executed in 10 s increments of 0.5°C with the temperature increasing from 55 to 95°C with the optics on. Effciency and consistency of the cDNA synthesis was determined by amplification of the human 18S gene as a control. For each round of amplifications, only those samples that were within 1.6 cycles respect to the mean for 18S were considered for further analysis. This allows for comparisons of different initial amounts of total RNA within a range of 10-fold differences represented by the 3.3 cycles in real time determinations.
Data analysis for real-time RT-PCR
Relative expression was determined using the 2−ΔΔCt method established by Livak and Schmittgen (29) with the use of three control genes for normalization. Each cycle threshold of target gene was normalized respect to the averaged value obtained for 18S, gliceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin-β (ACTB) (Table 2). Relative expression was calculated by comparing normalized cycle threshold values respect to the mean value of controls. Each individual sample including those of the control cases was analyzed respect to this mean value of controls using the 2−ΔΔCt algorithm giving a representative value of fold increase (higher than 1) or decrease (smaller than 1). Data is presented as mean ± standard error mean of fold increase respect to 1 (mean control value).
The following genes were analyzed according to their published accessible sequences of the Gene Bank: TNF-α (NM_000594); IL-1β (NM_000576); IL-6 (NM_000600); IL-4 (NM_000589); IL-5 (NM_00879); IL-13 (NM_002188), human ribosomal RNA subunit 18S (18S, K03432), GAPDH (NM_002046), ACTB (NM_001101).
Statistical analyses
Relative expression values of cytokines were logarithmically transformed to equalize standard deviations (SD) and produce normal distributions. Log transformed values for each cytokine were analyzed using the analysis of covariance (ancova) (30). The ancova model used consisted of cytokine expression values as dependent variable and suicide status, gender, age, and cause of death as independent variables. All tests were two-tailed with statistical significance set at alpha = 0.05. Statistical analyses were performed using sas version 9.1 (SAS Institute, Cary, NC, USA).
Results
Descriptive statistics of the sample
There were no differences between suicide victims and controls regarding the PMI gender or pH. However, the suicide victim group was slightly younger (52 years) (n = 34) than the control group (61 years) (n = 17). In suicide victims, data for blood alcohol (mg alcohol/g blood) was available for 82% of the cases (n = 28) whereas for the control group, data was only available for 17% of the cases (n = 3). Suicide victims tested positive for alcohol in 41% (n = 14) of the cases and negative in 38% of the cases (n = 13). Controls tested positive for alcohol in three cases from 17 and no information was available in the rest of the cases. Suicide victims included nine cases of major depression and one case of bipolar I disorder. Two cases had a history of alcohol abuse (AA). Psychological information was not available for the rest of the cases.
Identification of mRNA species for cytokines in brain tissue
Specific products using the real-time RT-PCR amplification conditions described were detected for all the set of primers. Figure 1 shows a 1.8% agarose gel electrophoresis of final products after 40 cycles of real-time PCR reaction. Single bands corresponding to specific cytokines can be observed on top of the band corresponding to the primers. The image also shows that when the reverse transcriptase enzyme step was omitted (RT−), no product was detected ruling out potential genomic contamination and confirming that the product originated from an RNA specie. The only exception to this rule was the band corresponding to IL-5. Due to the size of the amplified product (62 base pairs, Table 2), the product merged with the band of the primers. We confirmed amplification of this product by cloning and sequencing as described in methods.
Fig. 1.
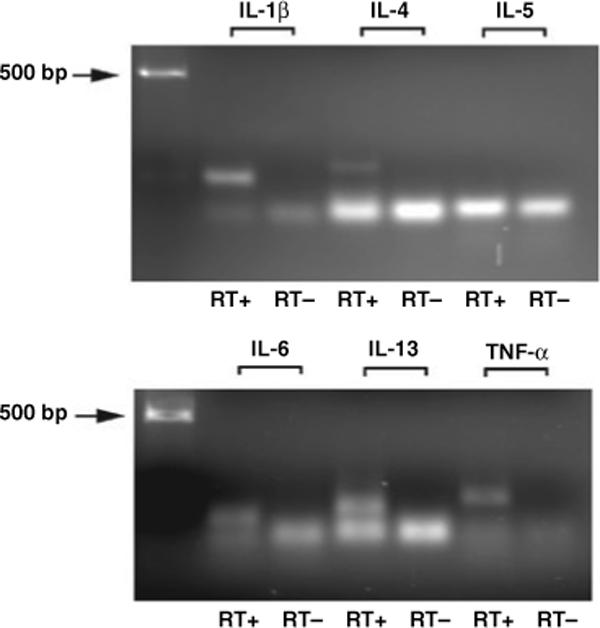
Agarose gel electrophoresis images of amplified products after 40 cycles of real-time RT-PCR reaction using the set of primers listed in Table 2. Single bands corresponding to specific cytokines can be observed on top of the primer-dimer bands. RT+: reaction containing cDNA originated from total RNA. RT−: no amplification of product when RNA was amplified without reverse transcriptase step.
Specific products were detected for all the cytokines and for all the cases analyzed. Melting curves showed single peaks in all the cases.
Cytokine expression in Brodman area 11
Analysis of the real-time data obtained for cytokines showed that the ratio of mRNA expression of cytokines to that of the housekeeping genes was constant between controls and suicide victims indicating that the data was suitable for further analysis. Using the averaged value for the three control housekeeping genes the ratios of expression for cytokines considering controls and suicides were as follows: 2.12 ± 0.01 for IL-13; 2.25 ± 0.01 for IL-1; 2.26 ± 0.01 for TNF-α; 2.27 ± 0.02 for IL-6 and 2.3 ± 0.01 for IL-4 and IL-5.
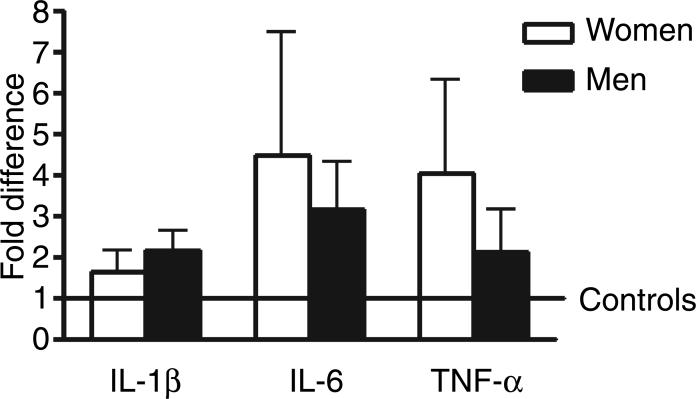
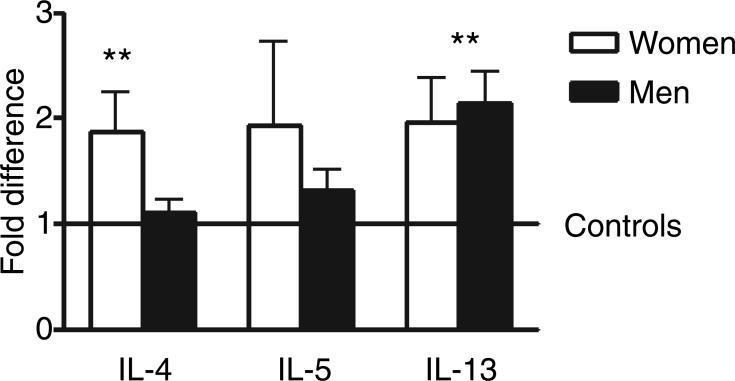
Analysis of covariance for the classical brain cytokines, IL-1β, IL-6, and TNF-α showed no significant difference between controls and suicides and no effect was detected for gender (Fig. 2). On the other hand, the ancova revealed significant differences for IL-4 between suicide victims and controls (F = 6.51; P = 0.0136) and between women and men (F = 30.3; P < 0.0001). The estimated ratio of geometric means of suicide victims vs. control was 1.764 and the ratio of men vs. women was 0.326 with increased values in women victims of suicide (Fig. 3). Similarly, a significant difference between suicides and controls were observed for IL-13 (F = 14; P = 0.0004) (Fig. 3) and between women and men (F = 4.38; P = 0.041). The estimated ratio of geometric means of suicide vs. control was 2.240 and the ratio of men vs. women was 1.512. No significant differences were found for IL-5. No effects were detected for any cytokine when considering age and cause of death.
Fig. 2.
Relative mRNA expression levels of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) in Brodman area 11 of suicide victims with respect to controls that died of other causes. Values are fold increase ±SEM respect to the mean of controls (represented by the number 1).
Fig. 3.
Relative mRNA expression levels of interleukin-4 (IL-4), IL-5, and interleukin-13 (IL-13) in Brodman area 11 of suicide victims with respect to controls that died of other causes. Values are fold increase ±SEM respect to the mean of controls (represented by the number 1) (**P < 0.001 respect to control).
Further analyses of individual cases revealed that in women victims of suicide, ten cases of 14 showed increased values compared to control for IL-4 (Table 3) with six of them having also increased values of IL-6. Moreover, in men 13 cases of 20 showed increased values for IL-13 with seven of them also having elevated TNF-α values. However, no significant correlations among cytokines were detected. The number of suicide cases showing increased, no change or decreased values respect to controls for all the cytokines are shown in Table 3.
Table 3.
Total number of suicide victims showing increased, no change or decreased expression respect to controls for all the cytokines analyzed
| Suicides | IL-1β | IL-6 | TNF-α | IL-4 | IL-5 | IL-13 |
|---|---|---|---|---|---|---|
| Women (n =14) | ||||||
| Increased | 7 | 10 | 7 | 10 | 5 | 8 |
| No change | 3 | 0 | 2 | 3 | 6 | 4 |
| Decreased | 4 | 4 | 5 | 1 | 3 | 2 |
| Men (n = 20) | ||||||
| Increased | 12 | 9 | 10 | 7 | 7 | 13 |
| No change | 3 | 2 | 3 | 4 | 7 | 4 |
| Decreased | 5 | 9 | 7 | 7 | 6 | 3 |
TNF-α, tumor necrosis factor alpha; IL, interleukin.
Discussion
The present study is the first to document and compare the presence and relative expression of mRNA species for cytokines in human cortex in psychiatric non-neurological cases. Moreover, the present study reports for the first time increased levels of type 2 T-helper cytokines in suicide victims. These results provide additional evidence of the potential participation of inflammatory processes in the brain, in particular cytokines, in suicide.
The orbitofrontal cortex including Brodman area 11 has been strongly implicated as a structure associated with inhibition of aggression, impulsivity and suicidal behavior (16, 31, 32). In addition, postmortem studies have demonstrated neurotransmitter abnormalities in this area of the brain of suicides such as decreased serotonin transporter binding sites and increased 5-HT1A and 5-HT2A receptor binding (32, 33). These findings are also consistent with functional imaging studies that demonstrate decreased activity in the ventral prefrontal cortex associated with suicidal and aggressive behaviors (17, 31, 34). In sum, there is strong evidence from functional imaging and postmortem studies implicating the orbitofrontal cortex, including Brodman area 11, in inhibiting or modulating many pro-suicidal factors including hopelessness, impulsivity, aggression, and anxiety. Therefore, the presence of mRNA for cytokines in this region of the human cortex may be of relevance because it indicates local production of molecules with potential to influence important brain functions related to suicidal behavior.
Cytokines are regulatory peptides that participate in host defense and repair processes of tissues. In the brain, the most studied cytokines are IL-1β, TNF-α, and IL-6 and have been implicated in a variety of normal and pathological conditions (2). Among many roles, they modulate neuroendocrine functions, sleep, sickness behavior and participate in neuroinflammatory and neurodegenerative processes (2). They have also been implicated in the neurobiology of mood disorders (1–4, 13, 14). Data on the functions of IL-4, IL-5, and IL-13 in the brain is less available. It has been reported that they counter-balance pro-inflammatory processes with potential beneficial effects in experimental models of autoimmune encephalomyelitis (35). However, studies using cultured microglial cells have shown effects of activation and production of inflammatory mediators in response to these cytokines (36–38). In addition, there is compelling evidence indicating that these cytokines may be involved in the neuropathology of schizophrenia (39, 40). The present study showing increased levels of IL-4 and IL-13 transcription in the orbitofrontal cortex of suicides suggests that these cytokines may affect neurobehavioral processes relevant to suicide. Nevertheless, the present study cannot address if the expression of cytokines is secondary to presuicidal anguish or if it is a factor of vulnerability predisposing individuals to suicide.
Several mechanisms have been proposed to explain how cytokines may affect brain function and behavior. Among them, the most studied are interactions with the hypothalamic–pituitary–adrenal (HPA) axis and with the enzyme indoleamine-2,3-dioxygenase (IDO). In the first case, cytokines can induce activation of the HPA-axis resulting in altered cortisol levels with detrimental effects for neurons. The latter relates to the activation or inhibition of the IDO enzyme that will ultimately result in altered serotonin metabolism and production of neuroactive substances. For reviews on these issues see (39–42).
It has been reported that allergies which involve the production of IL-4 and IL-13 are associated with increased suicide rates in women (19, 23). The present findings for IL-4 in women suicide victims are consistent with these epidemiological studies. Moreover, elevated IL-13 in suicide women was observed but the differences were not significant. The finding of increased IL-13 in suicide men may be related to several other factors including those related to the reported gender effects in suicidal behavior (43, 44) and the specific functions of this cytokine (45). In this regard, it should be highlighted that while there is abundant information about gender differences in suicide risk factors and suicidal behavior, to our knowledge, postmortem studies have not reported major neurochemical or gene expression differences between the brain of suicides women and men. The observed sex-differences in cytokine expression in the present study may be of relevance because sex-differences in the immune and cytokine response of the brain have been reported in experimental animal models (46–48). Therefore, cytokines may further our understanding of neurochemical sex-differences in the brain that may be related to sex-differences in behavior.
The present study contains several limitations that should be considered when interpreting the current results. The most important are non-matched controls for age, incomplete toxicology (82% of cases), and limited information on psychological diagnosis (26%). In the first case, a correlation between cytokine expression and age was not detected, a finding consistent with the study of Steiner et al. (12). However, for the cases of alcohol and suffering from a psychiatric condition, the present study may not represent accurate interactions of these factors with the expression of cytokines. This limitation is relevant considering the reported effects of alcohol on inflammatory processes in the brain (49, 50) and the association between cytokines and psychiatric diseases (40, 41). Future studies matching controls and suicides for these factors, expanding the analysis to other brain regions, and including additional cytokines will be necessary to strengthen the evidence on the involvement of cytokines in suicide. If so, studies aimed at determining the cellular origin of mRNA species for cytokines in the brain may become of relevance.
If further corroborated in larger samples, the present results may contribute to identify previously unexplored factors that may participate in the pathophysiology of suicide.
Acknowledgements
This study was supported by the American Foundation for Suicide Prevention and NARSAD Independent Investigator Award to Dr Postolache. Drs Tonelli and Postolache were additionally supported by R21 MH075891 (PI Postolache, CoPI Tonelli), R21 MH075905 (PI Tonelli, CoPI Postolache), and R01MH074891 (PI Postolache). We thank Andreas Thalmeier and Jim Soriano, MS, for their assistance.
References
- 1.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 2.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- 3.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Baron DA, Hardie T, Baron SH. Possible association of interleukin-2 treatment with depression and suicide. J Am Osteopath Assoc. 1993;93:799–800. [PubMed] [Google Scholar]
- 6.Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 7.Fukunishi K, Tanaka H, Maruyama J, et al. Burns in a suicide attempt related to psychiatric side effects of interferon. Burns. 1998;24:581–583. doi: 10.1016/s0305-4179(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 8.Gaudin JL, Faure P, Godinot H, Gerard F, Trepo C. The French experience of treatment of chronic type D hepatitis with a 12-month course of interferon alpha-2B. Results of a randomized controlled trial. Liver. 1995;15:45–52. doi: 10.1111/j.1600-0676.1995.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 9.Lana-Peixoto MA, Teixeira AL, Jr, Haase VG. Interferon beta-1a-induced depression and suicidal ideation in multiple sclerosis. Arq Neuropsiquiatr. 2002;60:721–724. doi: 10.1590/s0004-282x2002000500007. [DOI] [PubMed] [Google Scholar]
- 10.Nassberger L, Traskman-Bendz L. Increased soluble inter-leukin-2 receptor concentrations in suicide attempters. Acta Psychiatr Scand. 1993;88:48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- 11.Rothenhausler HB, Stepan A, Kapfhammer HP. Soluble interleukin 2 receptor levels, temperament and character in formerly depressed suicide attempters compared with normal controls. Suicide Life Threat Behav. 2006;36:455–466. doi: 10.1521/suli.2006.36.4.455. [DOI] [PubMed] [Google Scholar]
- 12.Steiner J, Bielau H, Brisch R, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli LH, Postolache TT, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Front Biosci. 2005;10:675–680. doi: 10.2741/1562. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- 15.Gosselin D, Rivest S. Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain Behav Immun. 2007;21:281–289. doi: 10.1016/j.bbi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 17.Oquendo MA, Placidi GP, Malone KM, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 19.Postolache TT, Stiller JW, Herrell R, et al. Tree pollen peaks are associated with increased nonviolent suicide in women. Mol Psychiatry. 2005;10:232–235. doi: 10.1038/sj.mp.4001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timonen M, Jokelainen J, Hakko H, et al. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry. 2003;8:738–744. doi: 10.1038/sj.mp.4001274. [DOI] [PubMed] [Google Scholar]
- 21.Timonen M, Jokelainen J, Herva A, Zitting P, Meyer-Rochow VB, Rasanen P. Presence of atopy in first-degree relatives as a predictor of a female proband's depression: results from the Northern Finland 1966 Birth Cohort. J Allergy Clin Immunol. 2003;111:1249–1254. doi: 10.1067/mai.2003.1546. [DOI] [PubMed] [Google Scholar]
- 22.Timonen M, Jokelainen J, Silvennoinen-Kassinen S, et al. Association between skin test diagnosed atopy and professionally diagnosed depression: a Northern Finland 1966 Birth Cohort study. Biol Psychiatry. 2002;52:349–355. doi: 10.1016/s0006-3223(01)01364-6. [DOI] [PubMed] [Google Scholar]
- 23.Timonen M, Viilo K, Hakko H, et al. Is seasonality of suicides stronger in victims with hospital-treated atopic disorders? Psychiatry Res. 2004;126:167–175. doi: 10.1016/j.psychres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Kingsbury AE, Foster OJ, Nisbet AP, et al. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- 25.Bahn S, Augood SJ, Ryan M, Standaert DG, Starkey M, Emson PC. Gene expression profiling in the post-mortem human brain–no cause for dismay. J Chem Neuroanat. 2001;22:79–94. doi: 10.1016/s0891-0618(01)00099-0. [DOI] [PubMed] [Google Scholar]
- 26.Johnston NL, Cervenak J, Shore AD, Torrey EF, Yolken RH. Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT-PCR. Stanley Neuropathology Consortium. J Neurosci Methods. 1997;77:83–92. doi: 10.1016/s0165-0270(97)00115-5. [DOI] [PubMed] [Google Scholar]
- 27.Yasojima K, McGeer EG, McGeer PL. High stability of mRNAs postmortem and protocols for their assessment by RT-PCR. Brain Res Brain Res Protoc. 2001;8:212–218. doi: 10.1016/s1385-299x(01)00119-2. [DOI] [PubMed] [Google Scholar]
- 28.Mimmack ML, Brooking J, Bahn S. Quantitative polymerase chain reaction: validation of microarray results from postmortem brain studies. Biol Psychiatry. 2004;55:337–345. doi: 10.1016/j.biopsych.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Fleiss JL. Design and analysis of clinical experiments. John Wiley & Son Eds; New York: 1986. [Google Scholar]
- 31.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 32.Arango V, Underwood MD, Mann JJ. Postmortem findings in suicide victims. Implications for in vivo imaging studies. Ann N Y Acad Sci. 1997;836:269–287. doi: 10.1111/j.1749-6632.1997.tb52365.x. [DOI] [PubMed] [Google Scholar]
- 33.Oquendo MA, Russo SA, Underwood MD, et al. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Blair KS, Smith BW, Mitchell DG, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aharoni R, Teitelbaum D, Leitner O, Meshore A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci U S A. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzumura A, Sawada M, Itoh Y, Marunouchi T. Interleukin-4 induces proliferation and activation of microglia but suppresses their induction of class II major histocompatibility complex antigen expression. J Neuroimmunol. 1994;53:209–218. doi: 10.1016/0165-5728(94)90031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liva SM, de Vellis J. IL-5 induces proliferation and activation of microglia via an unknown receptor. Neurochem Res. 2001;26:629–637. doi: 10.1023/a:1010983119125. [DOI] [PubMed] [Google Scholar]
- 38.Yang MS, Ji KA, Jeon SB, et al. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: implications for death of activated microglia. J Immunol. 2006;177:1323–1329. doi: 10.4049/jimmunol.177.2.1323. [DOI] [PubMed] [Google Scholar]
- 39.Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- 40.Muller N, Schwarz MJ. Neuroimmune-endocrine crosstalk in schizophrenia and mood disorders. Expert Rev Neurother. 2006;6:1017–1038. doi: 10.1586/14737175.6.7.1017. [DOI] [PubMed] [Google Scholar]
- 41.Capuron L, Bluthe RM, Dantzer R. Cytokines in clinical psychiatry. Am J Psychiatry. 2001;158:1163–1164. doi: 10.1176/appi.ajp.158.7.1163. [DOI] [PubMed] [Google Scholar]
- 42.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Wunderlich U, Bronisch T, Witchen HU, Carter R. Gender differences in adolescents and young adults with suicidal behavior. Acta Psychiatr Scand. 2001;104:332–339. doi: 10.1034/j.1600-0447.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- 44.Oquendo MA, Bongiovi-Garcia ME, Galfalvy H, et al. Sex differences in clinical predictors of suicidal acts after major depression: a prospective study. Am J Psychiatry. 2007;164:134–141. doi: 10.1176/appi.ajp.164.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 46.Barna M, Komatsu T, Bi Z, Reiss CS. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 47.Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- 48.Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301488. [Epub ahead of print].
- 49.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 50.Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]