Abstract
Pregnancy in women with mechanical valve prostheses has a high maternal complication rate including valve thrombosis and death. Coumarin derivatives are relatively safe for the mother with a lower incidence of valve thrombosis than un-fractionated and low-molecular-weight heparin, but carry the risk of embryopathy, which is probably dose-dependent. The different anticoagulation regimens are discussed in this review. When valve thrombosis occurs during pregnancy, thrombolysis is the preferable therapeutic option. Bioprostheses have a more favourable pregnancy outcome than mechanical prostheses but due to the high re-operation rate in young women they do not constitute the ideal alternative. When women with native valve stenosis need pre-pregnancy intervention, mitral balloon valvuloplasty is the best option in mitral stenosis, while the Ross operation or homograft implantation may be the preferable surgical regimen in aortic stenosis. (Neth Heart J 2008;16:406-11.)
Keywords: women, pregnancy, heart valve prosthesis
Worldwide many prosthetic valves are yearly implanted in girls and young women with rheumatic or congenital heart disease. Sooner or later many of them wish to become pregnant. Mechanical prostheses, bioprostheses and native valve disease each carry specific risks during pregnancy. These may affect the timing and type of surgical therapy. Pre-pregnancy counselling as well as adequate monitoring and treatment when pregnancy is achieved are challenging tasks for cardiologists who care for these young women.
Physiological changes during pregnancy
During pregnancy plasma volume and cardiac output increase by 45 to 50%.1,2 At 16 weeks 80% of the cardiac output increase has been achieved, mainly due to an increase in stroke volume. Heart rate starts to rise early in pregnancy and continues to rise until the 32nd week of pregnancy, when the maximum increase of 10 to 20 beats per minute is achieved. Echocardio-graphic cardiac chamber dimensions increase by 2 to 5 mm during pregnancy.3 Systemic vascular resistance decreases due to the low resistance in the uterine vessels and elevated levels of vasodilators. This is accompanied by a fall in systemic blood pressure during the second trimester.4,5 Renal blood flow and glomerular filtration rate increase.6 Renin, angiotensin, atrium natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels all increase during normal pregnancy. Pregnancy induces a hypercoagulable state, due to increased plasma concentration of fibrinogen, factors VII, VIII, and X, and plasminogen activator inhibitor. Platelet adhesiveness is increased as well. In addition, resistance to activated protein C occurs.7 Also, in supine position venous blood flow in the legs is reduced due to inferior caval vein compression by the pregnant uterus, which further contributes to the tendency for hyper-coagulation.
Mechanical valve prosthesis
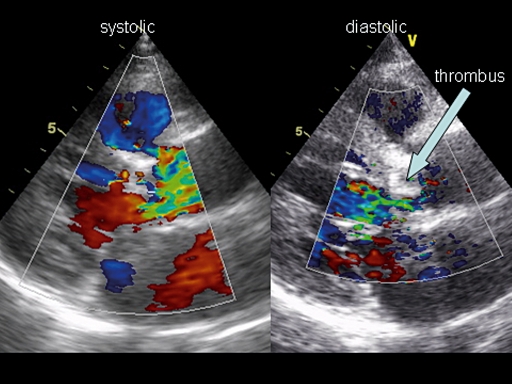
The hypercoagulability of pregnancy causes an increased incidence of mechanical valve thrombosis (figure 1). With any anticoagulation regimen adequate anticoagulation is more difficult to achieve during pregnancy. However, the incidence of valve thrombosis is lower with oral anticoagulants than with un-fractionated or low-molecular-weight heparin. On the other hand, foetal risk is higher with oral anticoagulants than with heparins.
Figure 1.
Björk Shiley mechanical valve with large thrombus.
Coumarin derivatives
Coumarin derivatives cross the placenta. Embryopathy due to coumarin derivative exposure was first recognised in 1965.8 The congenital abnormalities that have been described are midfacial hypoplasia as well as stippling of epiphyses (ectopic calcifications) on X-ray. In addition a wide variety of central nervous system abnormalities have been reported, ranging from hydrocephalus to optic atrophy. Coumarin derivatives are vitamin K antagonists and both bone/cartilage and the developing nervous system contain vitamin K dependant proteins, which may be a key to understanding the pathogenesis of the coumarin derivative embryopathy.9 A review describing 17 studies published from 1982 to 1999 describes 979 pregnancies with coumarin exposure.9 Warfarin was used in 33% and acenocoumarol in 46%; in the remaining women the coumarin derivative was not specified. In mothers using the coumarin derivative throughout pregnancy, the prevalence of skeletal abnormalities was 6% in 394 live-born children. In the offspring of women who used heparin from the 6th to the 12th week of pregnancy no skeletal abnormalities were reported.9 In 689 live-born children the estimated prevalence of central nervous system malformations was 1%, and 12 of the 13 children with these abnormalities had been exposed to coumarin derivatives during organogenesis. One small retrospective study of 58 pregnancies suggests that the risk of coumarin embryopathy may be dose-dependent, with no embryopathy in 33 children whose mother was on <5mg warfarin daily, while 2/25 children had embryopathy when the warfarin dose was >5 mg.10 This study, however, is too small to draw definite conclusions. The embryopathy incidence of 6% mentioned in several reviews9,11 possibly overestimates the current risk, as higher coumarin doses were used in the USA in the 1960s and 1970s and many of the included pregnancies took place in that period. More recent studies tend to report a lower incidence.12
Foetal loss appears to be high both when coumarin derivatives are used throughout pregnancy and with substitution of heparin during the first trimester, and differs from 25 to 70%, with a mean incidence of 34% in a large review.11 It must be noted that foetal loss is also high when heparin is used throughout pregnancy.
There have been concerns about a higher incidence of neurological dysfunction in children with coumarin exposure. In a large cohort study investigating the long-term neurological development of children with coumarin derivative exposure during pregnancy, only minor neurological dysfunctions were found more frequently compared with non-exposed children (OR 1.9,95%CI1.1to3.4).9
Thromboembolic complications were reported in a large systematic review by Chan et al. in 3.9% (788 pregnancies) with oral coagulants throughout pregnancy and in 9.2% (229 pregnancies) with substitution of unfractionated heparin in the first trimester;11 maternal mortality with these regimens was 1.8 and 4.2%, respectively, and in the majority valve thrombosis was the cause of death.
Unfractionated heparin
Unfractionated heparin does not cross the placenta. Therefore, the risk of embryopathy is eliminated with the use of heparin. Heparin is, however, associated with more maternal complications than warfarin. Thrombocytopenia may occur. In pregnant women using heparin for a mean duration of 17 weeks osteo-porotic vertebral fractures occurred in 2.2%.13 In women with mechanical prosthetic valves the main concern is valve thrombosis. In the systematic review mentioned above the incidence of thromboembolic events was 33% in 21 women who used unfractionated heparin throughout pregnancy.11 However, both for these 21 women and for the 229 women using heparin in the first trimester, the high rates of valve thrombosis were possibly related to inadequate dosing. Another limitation of this review is the non-contemporary high number of patients with older types of more thrombo-genic valve prostheses. Both subcutaneous and intravenous heparin were used in the studies of this review.
Another study reported 92 pregnancies, in 31 pregnancies dose-adjusted subcutaneous heparin was given in the first trimester (APTT twice the control level at four hours after dosing).14 Thromboembolic episodes were observed in four of these pregnancies, but these were all minor transient cerebral events and no obstructive valve thrombosis occurred. With warfarin throughout pregnancy only two minor episodes occurred in 61 pregnancies. Foetal loss was comparable with both regimens. This study indicates that adequate heparin dosing may reduce the number of serious thromboembolic events.
Low-molecular-weight heparins
The concerns about embryopathy with coumarin derivatives and about mechanical valve thrombosis with unfractionated heparin have raised interest in the use of low-molecular-weight heparins (LMWH) in pregnant women with mechanical valves. LMWH do not cross the placenta and outside pregnancy stable anticoagulation is more easily achieved than with unfractionated heparin. Other advantages of LMWH may be a lower incidence of osteoporosis and thrombocytopenia compared with unfractionated heparin.
In 2004 a review reported 81 pregnancies in 75 women.15 In 21 pregnancies LMWH were used during the first trimester, in the other pregnancies LMWH were used throughout pregnancy. In 51 pregnancies dose adjustment to maintain therapeutic anti-Xa levels was performed; a fixed dose was used in 30 pregnancies. In ten pregnancies thromboembolic events were reported, seven of these were valve thromboses in bileaflet valves. Nine of these ten thromboembolic events occurred in the 30 pregnancies with a fixed LMWH dose and only one in the 51 pregnancies with adjusted LMWH dose. All thromboembolic episodes occurred in women with mitral valves. Older studies with other anticoagulation regimens also reported a higher risk in mitral than in aortic valves. A South-African randomised controlled trial comparing unfractionated heparin with enoxaparin was prematurely discontinued because two women in the enoxaparin group died from valve thrombosis. Anti-Xa levels were monitored but no dose adjustment was performed for subtherapeutic levels.16 This study caused the manufacturer of enoxaparin to issue a warning against the use of enoxaparin in patients with mechanical valve prosthesis. This warning was later changed to a declaration that the use of enoxaparin in pregnant women with mechanical valves has not been adequately studied.
It appears from the above-mentioned studies that LMWH may be safe if dose adjustment according to anti-factor Xa levels is performed. It has been shown that dalteparin requirements increase in pregnancy. In 11 of 13 pregnancies, upward dosage adjustments were necessary to maintain adequate peak levels, and trough levels were in the therapeutic range only 9% of the time despite these dose adjustments.17 LMWH are cleared renally, therefore the increased glomerular filtration rate occurring in pregnancy contributes to the explanation of higher dose requirements. Another factor is probably the increase in plasma volume. A regimen of three rather than two daily dosages may be necessary in many women to achieve therapeutic peak and trough levels.
Aspirin
During pregnancy low-dose aspirin is a safe drug. Limited evidence suggests that in selected patients with mechanical valves outside pregnancy the addition of low-dose aspirin to coumarin derivatives results in less thromboembolic complications.18 Therefore it has been advocated that the addition of aspirin to coumarin derivatives or heparin can be considered in high-risk pregnant women with mechanical valves.19,20
Anticoagulation in women with mechanical valves: summary and current guidelines
The use of coumarin derivatives throughout pregnancy carries a risk of embryopathy of about 6%;
This risk is possibly lower with a warfarin dose <5 mg daily but no definite conclusions can be drawn; (a warfarin dose <5 mg daily probably corresponds with an acenocoumarol dose <2.5 mg but this may differ between individuals, therefore presumably a safe daily dose of acenocoumarol may be <2.0 mg);21
Substitution of coumarin derivatives with un-fractionated or low-molecular-weight heparin from the 6th to the 12th week of pregnancy eliminates the risk of embryopathy;
Coumarin derivatives appear to be safer for the mother with a lower incidence of thromboembolic events than unfractionated or low-molecular-weight heparin;
Required dosages during pregnancy for all anticoagulants can differ from dosages outside pregnancy. The risk of valve thrombosis with unfractionated or low-molecular-weight heparin is probably lower when aggressive dose-adjustment takes place, based on monitoring of APTT or anti-Xa levels;
All anticoagulation regimens are understudied and large prospective comparison is necessary.
Recommendations about the management of anti-coagulation in pregnant women with mechanical heart valves are included in recent guidelines of the American College of Cardiology/American Heart Association (ACC/AHA)19 and of the European Society of Cardiology (ESC).20 The level of evidence of these guidelines is C (i.e. based on consensus of experts and/or retrospective/small studies).
Both guidelines recommend continuing coumarin derivatives until pregnancy is achieved. The risks of different anticoagulation regimens must be considered and discussed. Treatment options when pregnancy is achieved include continuation of coumarin derivatives throughout pregnancy as well as dose-adjusted subcutaneous or intravenous unfractionated heparin between the 6th and the 12th week or throughout pregnancy with an APTT at least twice the control level. The ACC/AHA but not the ESC guidelines include the option of LMWH instead of unfractionated heparin with anti-Xa levels between 0.7 and 1.2 U/ml four hours after administration. Both guidelines include the advice to substitute coumarin derivatives a few weeks before planned delivery with unfractionated heparin. When delivery starts under oral anticoagulation Caesarean section is indicated because of the risk of intracranial bleeding in the anticoagulated baby with vaginal delivery. Table 1 provides a schedule with the most preferable regimen, in our opinion.
Table 1.
Anticoagulation regimen in pregnant women with mechanical prosthetic valves.
| Pre-pregnancy |
| – Discuss anticoagulation regimen with the patient |
| – Continue coumarin derivative until pregnancy is achieved |
| – When menstruation does not occur at expected day, perform pregnancy tests every 3 days until positive or until menstruation, in order to detect pregnancy at early stage |
| – Instruct patient to contact physician responsible for anticoagulation as soon as pregnancy is achieved |
| – Give patient and physician responsible for anticoagulation written instructions about anticoagulation regimen during pregnancy |
| 6th to 12th week of pregnancy |
| – If warfarin daily dose is <5 mg or acenocoumarol dose <2.0 mg, consider continuation of coumarin derivative throughout pregnancy |
| – Otherwise, substitute coumarin derivative with subcutaneous LMWH twice daily |
| – Adjust LMWH dose to achieve peak anti-Xa levels of 0.7 to 1.2 U/l ml 4 hours post dose |
| – If trough levels are subtherapeutic with therapeutic peak levels, dose 3 times daily |
| – Check anti-Xa levels weekly |
| 13th to 35th week of pregnancy |
| – Resume coumarin derivative |
| 36th week of pregnancy |
| – Substitute coumarin derivative with subcutaneous LMWH twice daily |
| – Adjust LMWH dose to achieve peak anti-Xa levels of 0.7 to 1.2 U/l ml 4 hours post dose |
| – If trough levels are subtherapeutic with therapeutic peak levels, dose 3 times daily |
| – Check anti-Xa levels weekly |
Alternatively, dose-adjusted unfractionated heparin to achieve APTT ≥ twice the control levels can be used instead of LMWH
Diagnosis and treatment of mechanical valve thrombosis during pregnancy
Presentation with dyspnoea or with an embolic event in patients with prosthetic valves must raise the suspicion of valve thrombosis. Immediate transthoracic echocardiography is indicated (figure 2); additional transoesophageal echocardiography (TEE) is usually necessary.22 If these examinations do not clearly confirm or rule out the diagnosis, fluoroscopy must be performed.22 Fluoroscopy can be regarded as relatively safe because the radiation dose for the foetus is very limited and unlikely to have adverse effects.23 Shielding of abdomen and pelvis is recommended. The 2007 ESC guidelines do not specify treatment of valve thrombosis in pregnancy. Outside pregnancy, the guidelines reserve fibrinolysis for critically ill patients when surgery is not immediately available and for patients with high surgical risk; in other cases surgery is advised as treatment of choice. Several authors, however, recommend fibrinolysis as the first choice in all patients with valve thrombosis.24,25 The ESC guidelines recommend that in selected cases (non-critically ill patients with recent inadequate anticoagulation or small non-obstructive thrombi) anticoagulation can be optimised first and if the thrombus disappears no further treatment is necessary.20 In pregnant women the risk of cardiac surgery for the mother is comparable with the risk outside pregnancy. However, there is a considerable risk of foetal loss (20 to 30%) associated with cardiopulmonary bypass.26,27 Thrombolysis has been used successfully in pregnant women with valve thrombosis without negative effects on the foetus, but only limited data are available.28 In pregnant women fibrinolysis is probably a safer option than surgery in most patients and should be considered. Streptokinase (100,000 U/h after a bolus of 250,000 U) orurokinase (4400 U/kg/h) can be given for a maximum of 72 hours, with three hourly transthoracic Doppler echocardiographic monitoring of thrombus resolve-ment. TEE should be performed after 24 hours and, if necessary, repeated at 48 and 72 hours.29 When surgery is necessary, deep hypothermia should be avoided whenever possible, because it is associated with foetal loss. Uterine contractions during surgery also predict foetal loss, therefore continuous monitoring of uterine contractions as well as of foetal heart rate is recommended.30
Figure 2.
Thrombosis of a St Jude aortic valve in a 28-week pregnant 30-year-old woman. Transthoracicpar asternal long-axis view. Note the narrow eccentric narrow systolic flow through the valve (left) and the eccentric turbulent valvular regurgitant flow (right). Peak pressure gradient was 70 mmHg.
Other risks
Thromboembolic episodes and embryopathy are not the only risks that must be faced by women with mechanical valves contemplating pregnancy. Symptomatic heart failure has been reported in patients with depressed systemic ventricular function and in patients who developed arrhythmias during pregnancy.31 Patient-prosthesis mismatch may also contribute to heart failure: both in aortic and mitral valve prosthesis a relation with heart failure was reported outside pregnancy.32,33 In addition a case of heart failure during pregnancy in a patient with patient-prosthesis mismatch was recently published.34 The risk of foetal complications including prematurity, low birth weight and mortality is also increased.35
Bioprosthesis
The risks of embryopathy and of valve thrombosis with considerable maternal mortality (estimated at 1 to 4% per pregnancy) raises the question whether mechanical valves should be implanted in girls and young women who need valve surgery. Is valve replacement with a bioprosthesis a better option in these patients? Maternal mortality during pregnancy is considerably lower in women with bioprostheses because the risk of valve thrombosis is avoided.36 Foetal outcome also appears to be better. The risk of heart failure and arrhythmias is probably comparable.37 However, young women with a bioprosthesis will almost certainly need re-operation, which is associated with a 4 to 9% mortality.35 This may well outweigh the risk of maternal mortality during pregnancy with a mechanical valve, especially because the rate of structural bioprosthetic valve degeneration is high in young women leading to the necessity for re-operation of 60 to 80% after ten years.12,35 The issue whether or not pregnancy accelerates the rate of structural valve deterioration has not been completely resolved due to conflicting study results. The high incidence of valve degeneration that has been reported during pregnancy could be due to the young age of the population.35 Structural valve deterioration rates are higher in mitral than in aortic bioprosthesis.12 Homografts probably have the advantage of better haemodynamics and lower valve deterioration rates.38,39 Maternal and foetal pregnancy complications also appear to be lower in women with homografts compared with heterografts.37 Only few pregnancies have been reported in women with a Ross operation, but cardiac complications seem to be limited.40,41 The re-operation rate with this procedure is lower than with aortic bioprosthesis, but higher than with mechanical valves. The Ross operation is complex and operation mortality was initially high, but is acceptable in later series. In experienced surgical hands, the Ross procedure can be an attractive alternative in women of child-bearing age with severe aortic valve disease.
Pre-pregnancy intervention in native valve disease
When a woman with native valve disease presents with the desire to become pregnant, a risk assessment must be performed in order to decide whether intervention is necessary before pregnancy. Stenotic lesions carry a higher maternal risk than regurgitant lesions. Pregnancy has a favourable maternal outcome in mild mitral stenosis. Moderate and severe mitral stenosis, however, are associated with maternal complications in 40 to 70%,42,43 but mortality is rare. Mild and moderate aortic stenosis is well tolerated. In severe aortic stenosis cardiac complications are reported in at least 10% with low mortality.44,45 However, though mitral and aortic stenosis are well recognised predictors of maternal adverse outcome,46 mechanical prosthesis has been reported to be an even stronger predictor of maternal complications in the Dutch ZAHARA study (results reported at the ESC congress 2007 by W. Drenthen). Bioprostheses are not an ideal alternative because of the mortality risk associated with the re-operation that is inevitable at young age. Women with severe mitral stenosis and symptomatic moderate mitral stenosis contemplating pregnancy should preferably be treated with percutaneous mitral balloon valvuloplasty before pregnancy.47 In aortic stenosis the Ross operation or homograft implantation may be a better option than a bioprosthesis. Mechanical valve implantation should be avoided whenever possible. In patients with severe mitral regurgitation surgical valvuloplasty should be considered; when the valve is not suitable for valvuloplasty and in aortic regurgitation pre-pregnancy surgery is usually not advisable.47
References
- 1.Pirani BB, Campbell, DM, MacGillivray I. Plasma volume in normal first pregnancy.J Obstet Gynaecol Br Commonw 1973;80:884-7. [DOI] [PubMed] [Google Scholar]
- 2.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol 1989;161:1439-42. [DOI] [PubMed] [Google Scholar]
- 3.Campos O. Doppler Echocardiography During Pregnancy: Physiological and Abnormal Findings. Echocardiography 1996;13:135-46. [DOI] [PubMed] [Google Scholar]
- 4.Capeless EL, Clapp JF. Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol 1989;161:1449-53. [DOI] [PubMed] [Google Scholar]
- 5.Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol 1997;272:H748-H752. [DOI] [PubMed] [Google Scholar]
- 6.Davison JM. Kidney function in pregnant women. Am J Kidney Dis 1987;9:248-52. [DOI] [PubMed] [Google Scholar]
- 7.Toglia MR, Weg JG. Venous thromboembolism during pregnancy. N Engl J Med 1996;335:108-14. [DOI] [PubMed] [Google Scholar]
- 8.Hall JG. Embryopathy associated with oral anticoagulant therapy. Birth Defects Orig Artic Ser 1976;12:33-7. [PubMed] [Google Scholar]
- 9.van Driel D, Wesseling J, Sauer PJ, Touwen BC, van der Veer E, Heymans HS. Teratogen update: fetal effects after in utero exposure to coumarins overview of cases, follow-up findings, and patho-genesis. Teratology 2002;66:127-40. [DOI] [PubMed] [Google Scholar]
- 10.Vitale N, De Feo M, De Santo LS, Pollice A, Tedesco N, Cotrufo M. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol 1999;33:1637-41. [DOI] [PubMed] [Google Scholar]
- 11.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000;160:191-6. [DOI] [PubMed] [Google Scholar]
- 12.Hung L, Rahimtoola SH. Prosthetic heart valves and pregnancy. Circulation 2003;107:1240-6. [DOI] [PubMed] [Google Scholar]
- 13.Dahlman TC. Osteoporotic fractures and the recurrence of thromboembolism during pregnancy and the puerperium in 184 women undergoing thromboprophylaxis with heparin. Am J Obstet Gynecol 1993;168:1265-70. [DOI] [PubMed] [Google Scholar]
- 14.Meschengieser SS, Fondevila CG, Santarelli MT, Lazzari MA. Anticoagulation in pregnant women with mechanical heart valve prostheses. Heart 1999;82:23-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.OranB, Lee-ParritzA, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost 2004;92:747-51. [DOI] [PubMed] [Google Scholar]
- 16.Elkayam U, Singh H, Irani A, Akhter MW. Anticoagulation in pregnant women with prosthetic heart valves. J Cardiovasc Pharmacol Ther 2004;9:107-15. [DOI] [PubMed] [Google Scholar]
- 17.Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol 2004;191:1024-9. [DOI] [PubMed] [Google Scholar]
- 18.Turpie AG, Gent M, Laupacis A, Latour Y, Gunstensen J, Basile F, et al. A comparison of aspirin with placebo in patients treated with warfarin after heart-valve replacement. N Engl J Med 1993;329:524-9. [DOI] [PubMed] [Google Scholar]
- 19.Bonow RO, Carabello BA, Chatterjee K, de Leon A Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Col Cardiol 2006;48:e1-148. [DOI] [PubMed] [Google Scholar]
- 20.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen Y, Rosendaal FR, van der Meer FJ. The relationship between maintenance dosages of three vitamin K antagonists: Acenocoumarol, warfarin and phenprocoumon. Thromb Res 2008 Apr 11 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Montorsi P, De Bernardi F, Muratori M, Cavoretto D, Pepi M. Role of cine-fluoroscopy, transthoracic, and transesophageal echo-cardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol 2000;85:58-64. [DOI] [PubMed] [Google Scholar]
- 23.Lowe SA. Diagnostic radiography in pregnancy: risks and reality. Aust N Z J Obstet Gynaecol 2004;44:191-6. [DOI] [PubMed] [Google Scholar]
- 24.Lengyel M. Thrombolysis should be regarded as first-line therapy for prosthetic valve thrombosis in the absence of contraindications. J Am Coll Cardiol 2005;45:325. [DOI] [PubMed] [Google Scholar]
- 25.Caceres-Loriga FM. Prosthetic valve thrombosis: is it time for a new consensus conference? Eur J Echocardiogr 2008;9:413-4. [DOI] [PubMed] [Google Scholar]
- 26.Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984-1996. Am J Obstet Gynecol 1998;179:1643-53. [DOI] [PubMed] [Google Scholar]
- 27.Arnoni RT, Arnoni AS, Bonini RC, de Almeida AF, Neto CA, Dinkhuysen JJ, et al. Risk factors associated with cardiac surgery during pregnancy. Ann Thorac Surg 2003;76:1605-8. [DOI] [PubMed] [Google Scholar]
- 28.Sahnoun-Trabelsi I, Jimenez M, Choussat A, Roudaut R. [Prosthetic valve thrombosis in pregnancy. A single-center study of 12 cases]. Arch Mal Coeur Vaiss 2004;97:305-10. [PubMed] [Google Scholar]
- 29.Lengyel M, Fuster V, Keltai M, Roudaut R, Schulte HD, Seward JB, et al. Guidelines for management of left-sided prosthetic valve thrombosis: a role for thrombolytic therapy. Consensus Conference on Prosthetic Valve Thrombosis. J Am Coll Cardiol 1997;30:1521-6. [DOI] [PubMed] [Google Scholar]
- 30.Parry AJ, Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg 1996;61:1865-9. [DOI] [PubMed] [Google Scholar]
- 31.Lesniak-Sobelga A, Tracz W, KostKiewicz M, Podolec P, Pasowicz M. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases—maternal and fetal outcome. Int J Cardiol 2004;94:15-23. [DOI] [PubMed] [Google Scholar]
- 32.Ruel M, Rubens FD, Masters RG, Pipe AL, Bedard P, Hendry PJ, et al. Late incidence and predictors of persistent or recurrent heart failure in patients with aortic prosthetic valves. J Thorac Cardiovasc Surg 2004;127:149-59. [DOI] [PubMed] [Google Scholar]
- 33.Lam BK, Chan V, Hendry P, Ruel M, Masters R, Bedard P, et al. The impact of patient-prosthesis mismatch on late outcomes after mitral valve replacement. J Thorac Cardiovasc Surg 2007;133:1464-73. [DOI] [PubMed] [Google Scholar]
- 34.Belford PM, Davis J, Wells G. A case of prosthesis-patient mismatch complicating pregnancy. Am J Perinatol 2007;24:241-2. [DOI] [PubMed] [Google Scholar]
- 35.Elkayam U, Bitar F. Valvular heart disease and pregnancy: part II: prosthetic valves. J Am Coll Cardiol 2005;46:403-10. [DOI] [PubMed] [Google Scholar]
- 36.Sbarouni E, Oakley CM. Outcome of pregnancy in women with valve prostheses. Br Heart J 1994;71:196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadler L, McCowan L, White H, Stewart A, Bracken M, North R. Pregnancy outcomes and cardiac complications in women with mechanical, bioprosthetic and homograft valves. BJOG 2000;107:245-53. [DOI] [PubMed] [Google Scholar]
- 38.North RA, Sadler L, Stewart AW, McCowan LM, Kerr AR, et al. Long-term survival and valve-related complications in young women with cardiac valve replacements. Circulation 1999;99:2669-76. [DOI] [PubMed] [Google Scholar]
- 39.Lund O, Chandrasekaran V, Grocott-Mason R, Elwidaa H, Mazhar R, Khaghani A, et al. Primary aortic valve replacement with allografts over twenty-five years: valve-related and procedure-related determinants of outcome. J Thorac Cardiovasc Surg 1999;117:77-90. [DOI] [PubMed] [Google Scholar]
- 40.Dore A, Somerville J. Pregnancy in patients with pulmonary autograft valve replacement. Eur Heart J 1997;18:1659-62. [DOI] [PubMed] [Google Scholar]
- 41.Yap SC, Drenthen W, Pieper PG, Moons P, Mulder BJ, Klieverik LM, et al. Outcome of pregnancy in women after pulmonary autograft valve replacement for congenital aortic valve disease. J Heart Valve Dis 2007;16:398-403. [PubMed] [Google Scholar]
- 42.Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol 2001;37:893-9. [DOI] [PubMed] [Google Scholar]
- 43.Silversides CK, Colman JM, Sermer M, Siu SC. Cardiac risk in pregnant women with rheumatic mitral stenosis. Am J Cardiol 2003;91:1382-5. [DOI] [PubMed] [Google Scholar]
- 44.Silversides CK, Colman JM, Sermer M, Farine D, Siu SC. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol 2003;91:1386-9. [DOI] [PubMed] [Google Scholar]
- 45.Yap SC, Drenthen W, Pieper PG, Moons P, Mulder BJ, Mostert B, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol 2008;126:240-6. [DOI] [PubMed] [Google Scholar]
- 46.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001;104:515-21. [DOI] [PubMed] [Google Scholar]
- 47.Elkayam U Bitar F. Valvular heart disease and pregnancy part I: native valves. J Am Coll Cardiol 2005;46:223-30. [DOI] [PubMed] [Google Scholar]