Abstract
Patients with congenital long-QT syndrome (LQTS) are at increased risk of ventricular arrhythmias during stressful situations. Large-scale studies have pointed out that affected individuals are particularly at risk in the period following pregnancy (post-partum). This is recognised especially for women with an LQTS type 2. Here, we describe two cases of young women with LQTS type 2, both admitted to our institution with symptomatic torsades de pointes a few weeks after delivery. Both patients carried a mutation in the KCNH2 gene. One patient was nullipara, while the other had had an uneventful previous pregnancy. In both cases treatment with a β-blocker did not prevent life-threatening cardiac arrhythmias. The risk of arrhythmias is thought to gradually decrease to pre-pregnancy values in the nine months after delivery. Considering the difficulties related to continuous monitoring of a patient for such a long period and the desire of these patients to have more children in the foreseeable future, ICD implantation was performed. (Neth Heart J 2008;16:422-5.)
Keywords: genetic, pregnancy, long-QT syndrome, beta-blocker
Long-QT syndrome (LQTS) is an electrical disorder of myocardial repolarisation characterised by a prolonged QT interval and associated with sudden cardiac death.1 It can be congenital, acquired or a combination of both. The prevalence of congenital LQTS is estimated at around 1:2000 and it is believed that congenital forms account for 3000 deaths of young people per year in the USA.2 Syncope and sudden death occur because of polymorphic ventricular tachycardia (VT) with specific features, known as torsades de pointes.3
Congenital forms are divided into types 1 to 10 according to the causal gene and the mode of transmission. Types 1,2 and 3 are the most common and together account for 95% of all congenital LQTS.2,4 They are well characterised and gene-specific ECG patterns as well as trigger factors for the occurrence of VT are established (table 1).5,6
Table 1.
Genes and gene products associated with the most prevalent LQTS types 1 to 3.
| Chromosome locus | Gene mutation/protein | Ion channel | Function of mutant | Relative frequency | Trigger factors | |
|---|---|---|---|---|---|---|
| LQTS 1 | 11p15.6 | KCNQ1 | IKs | Reduced | 45% | Exercise |
| LQTS 2 | 7q 35-36 | KCNH2 | IKr | Reduced | 35-40% | Emotion/noise |
| LQTS 3 | 3p 21-24 | SCN5A | INa | Increased | 8% | Sleeping/rest |
IKsand IKr designate the slow and rapid components, respectively, of the delayed rectifier potassium current. INa denotes the inward sodium current.
In LQTS type 2 stress and noise, presumably through an increased sympathetic activation, represent the most important trigger factors for the occurrence of torsades de pointes.7 Pregnancy and, in particular, the post-partum period are also associated with an augmented risk of cardiac events in all types of congenital LQTS, but especially in LQTS type 2.8 We describe two cases of patients with genetically demonstrated LQTS type 2, who both presented with syncope due to torsades de pointes within a few weeks after delivery.
Case1
A 27-year-old woman was being treated for LQTS type 2. She was diagnosed as part of family screening because her mother also had LQTS type 2. Her mother had been resuscitated following an appendectomy two months after giving birth. A heterozygous missense mutation V625E in gene KCNH2, encoding for the rapidly acting component of the delayed rectifier potassium current, was identified. The patient had suffered one syncopal episode at the age of 16; since then she had been stable on ?-blockers (metoprolol 50 mg/day), with a QTc of about 480 msec at routine ECGs.
An example of a routine ECG taken five years previously is shown in figure 1. In November 2006 she became pregnant. Metoprolol was continued and no events occurred during pregnancy. She was advised to have her baby in hospital. During delivery (continuous ECG monitoring was performed) no arrhythmias were documented. On the third day after delivery she was discharged from the hospital with unchanged medication. The QTc was longer than before pregnancy: 510 msec.
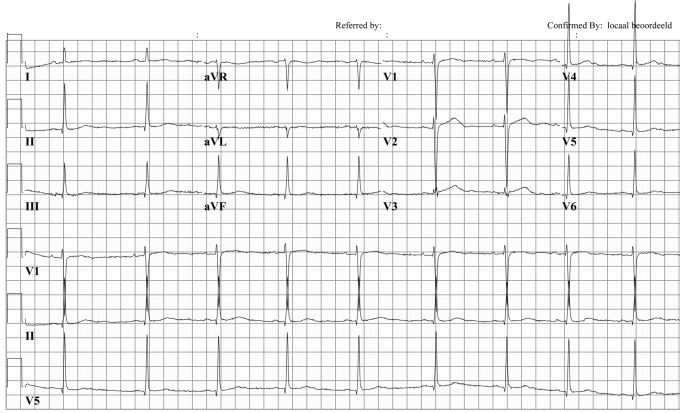
Figure 1.
Patient 1: Twelve-lead ECG taken in 2002 during routine examination. QTc: 480 msec. Note the low amplitude T waves in the inferior and lateral leads, which are typical for LQTS2 patients.
Four weeks later, she experienced syncope while standing upright at home. She was admitted with sinus rhythm, but after 24 hours she developed increasingly frequent episodes of torsades de pointes. The QT interval was extremely long (600 msec). The typical short-long-short RR interval sequence precedes torsades de pointes (figure 2). Laboratory tests revealed a serum K+ level of 3.8 mmol/l and a haemoglobin of 7.3 mmol/l.
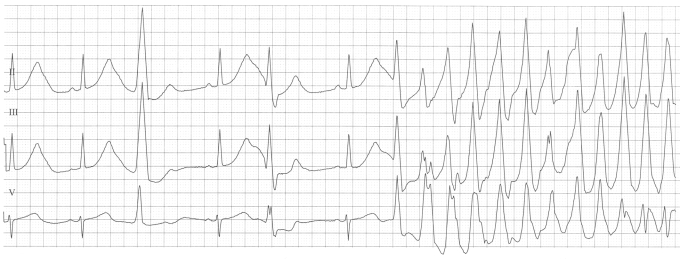
Figure 2.
Patient 1: ECG recording (leads II, III and V1 are shown) at admission after the patient had experienced syncope one month after delivery. Note the extremely long QT interval.
In the acute phase, she was treated with an infusion of magnesium sulphate, K+ suppletion and higher doses of β-blockers. An aldosterone antagonist was administered in order to keep K+ blood levels at approximately 5 mmol/l.9 Temporary transvenous pacing at a rate of 90 beats/min was necessary to prevent bradycardia-induced QT interval prolongation. Several hours later, her rhythm stabilised and no other episodes of torsades de pointes occurred. Because of documented VTs despite β-blockers, an ICD was implanted.
Case 2
A 32-year-old woman was diagnosed with LQTS in 1993 after an episode of syncope. A truncating mutation (E698X) in KCNH2 was found, establishing the diagnosis of LQTS type 2. She had been stable while on β-blockers (bisoprolol, which had been changed into metoprolol during pregnancy) for more than two years and, on routine ECGs, the QTc amounted to 430 msec. She had inherited the genetic alteration from her mother and her brother was also affected. The family history was negative for sudden cardiac death at young age. In 2004 she delivered a baby boy whose ECG was normal at birth. In August 2006 she delivered a baby girl and two months after delivery she experienced a syncope while on medication. ECG tracings showed multiple self-terminating torsades de pointes episodes (figure 3) and frequent ventricular premature beats that also occurred in doublets and triplets. QTc at that moment was prolonged up to 520 msec. She was treated with increased doses of β-blocker and K+ level was corrected (serum K+ level was 3.4 mmol/l). She underwent ICD implantation.
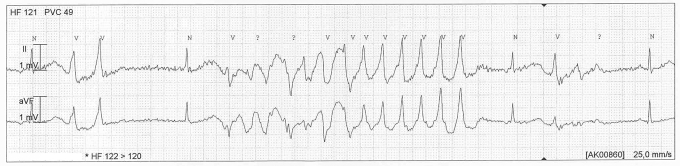
Figure 3.
Patient 2: Registration of an episode of self-terminating torsades de pointes in leads II and a VF. Calibration is shown.
Discussion
We describe here the cases of two young women with LQTS type 2, both suffering from life-threatening polymorphic VTs four to eight weeks after giving birth. Our experience confirms the findings of previously published large studies, that the post-partum period is associated with a significant increase in cardiac events in LQTS type 2 patients.8,10 The mechanism by which the highest risk clusters in the nine-month post-partum period remains unknown. Many co-factors potentially play a role and find their maximal expression in the days and weeks following delivery. Most likely this is due to changes in hormone balance (high levels of oestrogens and progesterone), fatigue and sleep deprivation, stress, noise (crying of the newborn infant), anaemia or a combination of the above. It seems plausible, as shown in our cases, that these factors reach their peak soon after delivery and that a gradual adaptation with a decrease in risk back to pre-pregnancy levels takes places in the course of the following months. It is not known whether there is also an association with breast feeding or the number of previous pregnancies.
Treatment with β-blockers, which has proven to significantly reduce the number of cardiac events in congenital LQTS patients, also during the post-partum period,8 should be continued. In our patients, this did not prevent the occurrence of potentially lethal arrhythmias. Interestingly, the affected mother of patient 1 had also been resuscitated two months after delivery. Although confounding factors were present (antibiotics, post-appendectomy), it may be hypothesised that a mutation-specific predisposition is present.
A close cardiac follow-up after pregnancy in LQTS2 is recommended. Guidelines dealing with this specific problem are lacking. In both cases QTc was increased compared with pre-pregnancy and pregnancy values. A practical suggestion could be to perform an ECG every one to two weeks after delivery and to observe the patient in a clinical setting when QT duration is significantly prolonged in comparison with pre-pregnancy values or when QTc exceeds 500 msec.
Conclusion
Pregnancy is a vulnerable period for the occurrence of cardiac events. This has now also been recognised for LQTS patients and especially for LQTS type 2. Our cases illustrate the importance of strict cardiac monitoring, not only before and during delivery, but particularly in the following weeks/months. In our opinion, a reasonable way to promptly recognise QT-interval prolongation, which accompanies the occurrence of VT, is to substantially increase the number of cardiological evaluations in the period after delivery.
References
- 1.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993;88:782-4. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med 2008;358:169-76. [DOI] [PubMed] [Google Scholar]
- 3.Jackman WM, Friday KJ, Anderson JL, Aliot EM, Clark M, Lazzara R. The long QT syndromes: a critical review, new clinical observations and a unifying hypothesis. Prog Cardiovasc Dis 1988;31:115-72. [DOI] [PubMed] [Google Scholar]
- 4.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 2000;102:1178-85. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 2001;103:89-95. [DOI] [PubMed] [Google Scholar]
- 6.Conrath CE, Jongbloed RJE, van Langen IM, van Tintelen, Hauer RNW, Robles de Medina EO, et al. Gene-specific distribution of cardiac events in LQTS1 and LQTS2. Neth Heart J 1999;6:254-9. [Google Scholar]
- 7.Wilde AA, Jongbloed RJ, Doevendans PA, Duren DR, Hauer RN, van Langen I, et al. Auditory stimuli as a trigger for arrhythmic events differentiate HERG-related (LQTS2) patients from KVLQT1-related patients (LQTS1). J Am Coll Cardiol 1999;33:327-32. [DOI] [PubMed] [Google Scholar]
- 8.Seth R, Moss AJ, McNitt S, Zareba W, Andrews ML, Qi M, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol 2007;49:1092-8. [DOI] [PubMed] [Google Scholar]
- 9.Tan HL, Alings M, Van Olden RW, Wilde AA. Long-term (sub-acute) potassium treatment in congenital HERG-related long QT syndrome (LQTS2). J Cardiovasc Electrophysiol 1999;10:229-33. [DOI] [PubMed] [Google Scholar]
- 10.Rashba EJ, Zareba W, Moss AJ, Hall WJ, Robinson J, Locati EH, et al. Influence of pregnancy on the risk for cardiac events in patients with hereditary long QT syndrome. LQTS Investigators. Circulation 1998;97:451-6. [DOI] [PubMed] [Google Scholar]