Abstract
Left ventricular assist devices (LVAD) are an effective therapeutic option for end-stage heart failure patients as a bridge to cardiac transplantation in those who deteriorate despite maximal therapy and when a donor heart is not ready available. In some patients, cardiac recovery has been reported while supported by an LVAD. In this case report, we describe a 29-year-old female who was admitted to our centre because of peripartum cardiomyopathy (PPCM). Despite intensive treatment with intravenous inotropes and intra-aortic balloon counter-pulsation she had a persisting low cardiac index and an LVAD was implanted. In the months following implantation the left ventricular systolic function improved and the left ventricular dimensions normalised. Eventually the LVAD could be ex-planted nine months after implantation. At this moment, three years after explantation, echo-cardiography shows a normal-sized left ventricle and almost completely recovered systolic function. (Neth Heart J 2008;16:426-8)
Keywords: peripartum cardiomyopathy (PPCM), left ventricular assist device (LVAD), cardiac transplantation, bridge to recovery
Peripartum cardiomyopathy (PPCM) is a form of acute heart failure which can have a fulminant course. The incidence of PPCM is approximately 1 per 3000 to 4000 pregnancies. Classic diagnostic criteria of PPCM are symptoms of heart failure developing one month prior to delivery until five months thereafter in patients without pre-existing heart disease, objective evidence of depressed left ventricular ejection fraction <45% and absence of other identifiable causes of heart failure.
The cause of PPCM is thought to be multifactorial. Risk factors associated with PPCM are: age >30 years, multiparity, multiple foetuses, history of preeclampsia, eclampsia or gestational hypertension, African descent and prolonged tocolysis. Prolactine may also play a role, as has been recently demonstrated in animal studies.1-4 Furthermore, autoimmunity and inflammation are potential causative factors, although the incidence of histologically proven myocarditis is variable. Mortality from severe heart failure is reported to be up to 20% in patients with PPCM, with the majority of deaths being sudden. Heart transplantation was performed in 4 to 10%.1,3 Spontaneous recovery of the left ventricular ejection fraction (LVEF) >50% was seen in 45 to 50% of patients. Recovery of the LVEF is mainly seen in the first two to six months after delivery but can improve over the first year.1,3
Predictors of complete recovery (EF >50%) are: left ventricular end-diastolic diameter <56 mm, mean left ventricular ejection fraction at two months after delivery >45% and the absence of thrombus in the left ventricle at the time of diagnosis.2 Medical therapy for PPCM consists mainly of regular heart failure therapy. In some cases intra-aortic balloon counterpulsation, inotropes and eventually heart transplantation is necessary.1,3
Case report
A 29-year-old female was admitted to a regional hospital and later transferred to a university hospital with symptoms of heart failure at 36 weeks' gestation. It was her second pregnancy, the first pregnancy had been uneventful. Echocardiography revealed a severely dilated left ventricle with an end-diastolic diameter of 85 mm, thin walls and a globally, severely diminished systolic function (figure 1). The mitral valve annulus was dilated resulting in regurgitation grade II-III / IV. The right ventricle showed a good function without dilatation; the right ventricular systolic pressure (RVSP) was slightly elevated to 45 mmHg. The diagnosis of PPCM was made based on symptoms of heart failure in the last month of pregnancy, objective evidence of depressed left ventricular ejection fraction and absence of other identifiable causes of heart failure. She underwent a caesarean section a couple of days after admittance because of progressive heart failure. Next, she was transferred to our hospital in anticipation of heart transplantation or mechanical circulatory support. She was treated with dopamine, milrinone, noradrenalin and intra-aortic balloon counterpulsation. Despite this extensive therapy, the low cardiac index persisted, with imminent multi-organ failure. The decision was made to implant a left ventricular assist device (HeartMate XVE). The implantation was uneventful. Endomyo-cardial biopsy at the time of implantation did not show arguments for myocarditis or other specific abnormalities. Six weeks after implantation of the left ventricular assist device (LVAD) the patient could be mobilised and discharged from the hospital. Two months after implantation she was placed on the waiting list for heart transplantation because echocardiography did not show any improvement in the left ventricular function. Medical therapy consisted of carvedilol 25 mg twice daily, enalapril 5 mg twice daily, and aspirin 100 mg once daily. In the following months, however, a gradual decrease in cardiac size and improvement of the left ventricular systolic function on echocardiographic examinations was noticed. To investigate recovery of left ventricular function, the LVAD was turned off for 30 minutes, while the patient was on full-dose heparin. Without mechanical support the left ventricular end-diastolic dimension was 56 mm and the left ventricular function was about normal, except for septal akinesia. The brain natriuretic peptide (BNP) plasma levels remained normal (11 pmol/l with and 13 pmol/l without support). To further substantiate left ventricular recovery, a second investigation was planned several weeks later in which haemodynamic measurements and exercise performance were assessed, while the LVAD was turned off for a couple of hours. Swan-Ganz catheterisation showed normal haemodynamic indices with and without support of the LVAD (PCW 7 mmHg, RA 3 mmHg, CO 5.6 l/min). The peak VO2 at exercise was 1793 ml/min or 22.4 ml/kg/min (82 and 60% respectively of predicted for her age) at a respiratory quotient (RQ) of 1.04, compatible with a normal exercise tolerance in daily life.
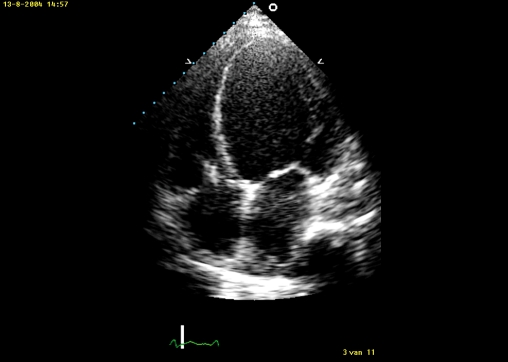
Figure 1.
AP4CH view before implantation of the left ventricular assist device showing a severely enlarged left ventricle with remarkable thinning of the left ventricular wall.
Because all the investigations demonstrated good recovery of left ventricular function, the decision was made to explant the LVAD. This was done nine months after implantation; the left ventricular function appeared good and the patient could be weaned from the heart-lung machine without problems. Because of the large left ventricular apical scar, due to the removed LVAD cannula, an ICD was implanted prophylactical-ly. Since explantation of the LVAD three years ago, the patient is doing well and she is in functional NYHA class I. She is still on carvedilol 25 mg twice daily and enalapril 5 mg twice daily. Recently, peak VO2 at exercise has increased to 2243 ml/min or 25.8 ml/kg/min (104 and 71% respectively of predicted for her age) at an RQ of 1.18. The left ventricular end-diastolic diameter remained stable at 56 mm with a fair to good systolic function of the left ventricle (LVEF 42%) (figure 2). There have been no ICD shocks.
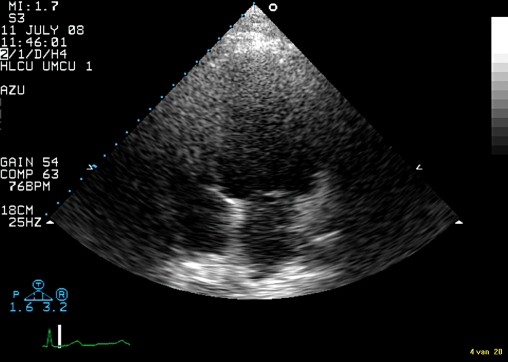
Figure 2.
AP4CH from July 2008 which shows a diminished left ventricular size although the technical quality is poor due to weight and scar tissue.
Discussion
In this report we describe a patient with end-stage heart failure with a severely dilated left ventricle due to peripartum cardiomyopathy (PPCM). Despite intensive inotropic therapy the haemodynamic situation deteriorated, necessitating the implantation of an LVAD. In the future, bromocriptine may play a role in the prevention and treatment of this disease, but this needs further investigation.4 In this patient bromocriptine was not started because at the time of diagnosis these data had not yet been published.
After LVAD implantation, left ventricular function improved substantially, allowing explantation of the device after nine months of circulatory support.
Many studies have demonstrated impressive improvement in survival, exercise performance and recovery of cardiac function after LVAD implantation. In general, cardiac dimensions decrease and on a cellular level, hypertrophy diminishes. Furthermore, many cellular processes improve, although they do not completely normalise.5-15
Sufficient recovery of left ventricular function, allowing LVAD explantation, however, is rare and is mainly feasible in patients suffering from acute myocarditis or PPCM.10,11,13
In these cases, the LVAD will maintain the patient's life, allowing the myocardium to recover from the underlying disease. Long-term results after explantation are favourable (one- and five-year survival rate of 86 and 78%, respectively).14,15
In patients with longstanding idiopathic dilating cardiomyopathy, the concept of reverse remodelling while on the LVAD is intriguing but requires further study to see to what extent this may result in explantation of the device, to investigate the optimal drug regime necessary and the reliable monitoring of this process.6-9
References
- 1.Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli T. Peripartum cardiomyopathy: A comprehensive review. Int J Cardiol 2007;118:295-303. [DOI] [PubMed] [Google Scholar]
- 2.Amos A, Jaber W, Russell S. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J 2006;152:509-13. [DOI] [PubMed] [Google Scholar]
- 3.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet 2006;368:687-93. [DOI] [PubMed] [Google Scholar]
- 4.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A Cathepsin D-Cleaved 16 kDa Form of Prolactin Mediates Postpartum Cardiomyopathy. Cell 2007;128:589-600. [DOI] [PubMed] [Google Scholar]
- 5.Lahpor J, de Jonge N, van Swieten H, Wesenhagen H, Klopping C, Geertman J, et al. Left ventricular assist device as bridge to transplantation in patients with end-stage heart failure / Eight year experience with the implantable HeartMate LVAD. Neth Heart J 2002;10:267-71. [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonge N, Van Wichen D, Schipper M, Lahpor J, Gmelig-Meyling F, Robles de Medina E, et al. Left Ventricular assist device in end-stage heart failure: persistence of structural myocyte damage after unloading /An immunohistochemical analysis of the contractile myofilaments. J Am Coll Cardiol 2002;39:963-9. [DOI] [PubMed] [Google Scholar]
- 7.Yacoub M. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J 2001;22:534-40. [DOI] [PubMed] [Google Scholar]
- 8.Dipla K, Mattiello J, Jeevanandam V, Houser S, Margulies K. Myocyte Recovery After Mechanical Circulatory Support in Humans With End-Stage Heart Failure. Circulation 1998;97:2316-22. [DOI] [PubMed] [Google Scholar]
- 9.Zafeiridis A, Jeevanandam V, Houser S, Margulies K. Regression of Cellular Hypertrophy After Left Ventricular Assist Device Support. Circulation 1998;98:656-62. [DOI] [PubMed] [Google Scholar]
- 10.Mancini D, Beniaminovitz A, Levin H, Catanese K, Flannery M, DiTullio M, et al. Low Incidence of Myocardial Recovery After Left Ventricular Assist Device Implantation in Patients With Chronic Heart Failure. Circulation 1998;98:2383-9. [DOI] [PubMed] [Google Scholar]
- 11.Simon M, Kormos R, Murali S, Mair P, Heffernan M, Gorcsan J, et al. Myocardial Recovery Using Ventricular Assist Devices / Prevalence, Clinical Characteristics, and Outcomes. Circulation 2005;112(suppl I):I-32-I-36. [DOI] [PubMed] [Google Scholar]
- 12.Birks E, Tansley P, Hardy J, George R, Bowles C, Burke M, et al. Left Ventricular Assist Device and Drug Therapy for the Reversal of Heart Failure. N Engl J Med 2006;355:1873-84. [DOI] [PubMed] [Google Scholar]
- 13.Maybaum S, Mancini D, Xydas S, Starling R, Aaronson K, Pagani F, et al. Cardiac Improvement During Mechanical Circulatory Support / A Prospective Mul ti center Study of the LVAD Working Group. Circulation 2007;115:2497-505. [DOI] [PubMed] [Google Scholar]
- 14.Farrar D, Holman W, McBride L, Kormos R, Icenogle T, Hendry P, et al. Long-Term Follow-up of Thoratec Ventricular Assist Device Bridge-to-Recovery Patients Successfully Removed From Support After Recovery of Ventricular Function. J Heart Lung Transplant 2002;21:516-21. [DOI] [PubMed] [Google Scholar]
- 15.Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl H, Hetzer R. Long-Term Results in Patients With Idiopathic Dilated Cardiomyopathy After Weaning From Left Ventricular Assist Devices. Circulation 2005;112(suppl I):I-37-I-45. [DOI] [PubMed] [Google Scholar]