Abstract
Abstract
During the last decennium, the role of bone marrow mononuclear cells (BMMC) has been underscored in the healing process after acute myocardial infarction (AMI). Although these cells improve left ventricular recovery after AMI in experimental studies, results from large-scale randomised trials investigating BMMC therapy in patients with AMI have shown contradictory results. To address this issue the HEBE study was designed, a multicentre, randomised trial, evaluating the effects of intracoronary infusion of BMMCs and the effects of intracoronary infusion of peripheral blood mononuclear cells after primary percutaneous coronary intervention. The primary endpoint of the HEBE trial is the change in regional myocardial function in dysfunctional segments at four months relative to baseline, based on segmental analysis as measured by magnetic resonance imaging. The results from the HEBE trial will provide detailed information about the effects of intracoronary BMMC therapy on post-infarct left ventricular recovery. In addition, further analysis of the data and material obtained may provide important mechanistic insights into the contribution of BMMCs to natural recovery from AMI as well as the response to cell therapy. This may significantly contribute to the development of improved cell-based therapies, aiming at optimising post-infarct recovery and preventing heart failure. (Neth Heart J 2008;16:436-9.)
Introduction
To date, coronary artery disease is the most frequent cause of death in the Western world. Despite optimal medical therapy and advanced revascularisation strategies, 30% of the patients suffer from post-infarct heart failure due to left ventricular remodeling.1 Ventricular remodeling induces profound changes, resulting in left ventricular dilatation and deterioration of function, which may lead to congestive heart failure.2 Prevention of this serious complication has become a major challenge. During the last decennium, the role of bone marrow mononuclear cells (BMMCs) has been underscored in the healing process after AMI. It has been shown that the number of circulating CD34+ BMMCs, including the haematopoietic stem cells and the endothelial progenitor cells, increases after AMI.3 These cells are recruited from the bone marrow to the infarcted area by chemokines such as stromal cell-derived factor-1.4 The spontaneous mobilisation of these cells from the bone marrow to the infarcted area may be an important reparative mechanism. It has been suggested that these cells contribute to infarct healing by paracrine effects, i.e. inducing angiogenesis, inhibiting apoptosis, and enhancing scar tissue formation, or possibly by myocardial regeneration.5,6
BMMC therapy: from experimental studies to clinical trials
In 2001, Orlic et al. reported that subsets of BMMCs are capable of replacing infarcted myocardium and improve ventricular function after AMI in mice.7 Moreover, a study by Quaini et al., investigating sex-mismatched transplanted hearts, demonstrated migration of cells from the recipient to the donor heart. Postmortem analysis of the donor heart showed that these cells had been residing in the myocardium, arterioles, and capillaries.8 Based on these findings, BMMCs were suggested to circulate in the blood and to replace damaged myocardial tissue after AMI. Promptly, small clinical trials of BMMC therapy in patients with AMI were initiated. The capability of BMMCs to replace damaged myocardial tissue is currently being questioned, because other laboratories were unable to reproduce the findings by Orlic et al. It seems more likely that the potential beneficial effects of BMMCs are induced by paracrine effects such as enhanced arterio-genesis and angiogenesis, following the release of angiogenic and arteriogenic cytokines. This hypothesis was strengthened by Kamihata et al.'s group showing an increased number of arterioles in response to peripheral blood mononuclear cells (PBMCs) and BMMC injection in the ischaemic myocardium of a pig model of AMI.9 After the first clinical trials of intracoronary infusion of BMMCs after AMI in patients showed the method to be safe and feasible,10,11 large-scale randomised trials were initiated to further evaluate the therapeutic effects of BMMC therapy. In the largest study so far, the REPAIR-AMI trial, 204 patients with AMI were randomly assigned to treatment with intracoronary infusion of BMMCs or placebo. At four months of follow-up, the improvement in left ventricular ejection fraction (LVEF) was significantly greater in the BMMC group than in the placebo group (5.5±7.3% vs. 3.0±6.5%;p=0.01), with a concomitant significant reduction in the combined endpoint death, MI and re-hospitalisation for heart failure at one year of follow-up.12,13 The ASTAMI trial, on the other hand, in which 100 patients with AMI were randomised to BMMC therapy or standard therapy, showed no beneficial effect of BMMC therapy on LVEF.14 So far, randomised trials of intracoronary infusion of BMMCs after AMI in patients have shown contradictory results. The reason for this contradiction is unclear and may be related to differences in methodology such as cell isolation procedures, timing of cell administration and dosage.15,16
The HEBE trial
To address this issue, the HEBE trial was designed in the Netherlands: a large, multicentre, randomised trial, evaluating the effects of intracoronary infusion of autologous BMMCs and the effects of intracoronary infusion of autologous PBMCs after primary percutaneous coronary intervention (PCI).17 Based on the study by Kamihata et al., the beneficial effects of the BMMCs can be attributed to the paracrine effects of all mononuclear cell subsets, rather than the progenitor subpopulation.18 For that reason, a patient group receiving intracoronary autologous PBMCs was included in the trial. A total of 200 patients with AMI treated with primary PCI with stent placement underwent magnetic resonance imaging (MRI) followed by intracoronary infusion of autologous BMMCs, intracoronary infusion of autologous PBMCs, or standard therapy (randomly assigned to a 1:1:1 ratio). After four months of follow-up, MRI was repeated. The primary endpoint of the HEBE trial is the change in regional myocardial function in dysfunctional segments at four months relative to baseline, based on segmental analysis as measured by MRI. Currently, the inclusion of patients in the HEBE trial has been completed (table 1), and the final results of the primary endpoint will be available at the end of 2008 (see page 439).
Table 1 .
Participating centres and inclusion per hospital.
| Participating hospitals | Inclusion |
|---|---|
| Academic Medical Center, Amsterdam | 58 |
| University Medical Center Groningen, Groningen | 87 |
| VU University Medical Center, Amsterdam | 18 |
| Erasmus University Medical Center, Rotterdam | 16 |
| University Medical Center Utrecht, Utrecht | 8 |
| University Hospital Maastricht, Maastricht | 6 |
| St Antonius Hospital, Nieuwegein | 5 |
| Radboud University Nijmegen Medical Centre, Nijmegen | 2 |
Pilot study of the HEBE trial
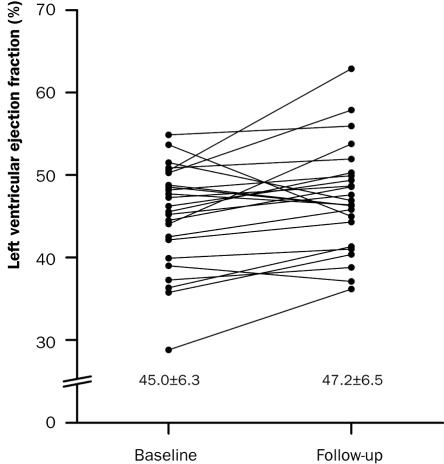
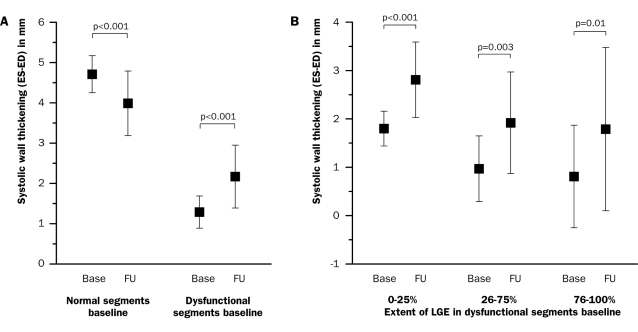
Prior to the HEBE trial, a pilot trial was performed to determine safety and feasibility of intracoronary infusion of BMMCs in patients with AMI. Twenty-six patients were prospectively enrolled in eight centres. At one year of follow-up, the major cardiovascular event rate was low. The LVEF significantly increased from 45.0±6.3% to 47.2±6.5% (p=0.03) (figure 1). Systolic wall thickening in dysfunctional segments at baseline improved by 0.9±0.7 mm (p<0.001). Improvement in systolic wall thickening was observed in all dysfunctional segments, including the most severely infarcted segments, as measured by MRI (figure 2). Infarct size decreased by 37% from 17.8±8.2 gram to 11.2±4.2 gram (p<0.001). It was concluded that intracoronary infusion ofBMMCs is safe and feasible in a multicentre setting. At four months of follow-up, a modest increase in global and regional left ventricular function was observed, with a concomitant decrease in infarct size.19
Figure 1.
The effect of intracoronary infusion ofBMMCs on LVEF after AMI. The LVEF significantly increased from 45.0±6.3% at baseline to 47.2±6.5% (p=0.03) at four months of follow-up as measured by MRI (modified from Hirsch et al. 19).
Figure 2.
Change in systolic wall thickening after intracoronary BMMC infusion in (A) dysfunctional segments at baseline versus normal segments (n=24) and in (B) dysfunctional segments stratified by extent of hyperenhancement (n=1 9). Improvement in systolic wall thickening was 1.0±0.6 mm in segments with 0-25% hyperenhancement, 0.9±1.2 mm in 26-75% and 1.0±1.4 mm in 76-100%. Base=baseline, ES=end-systolic, ED=end-diastolic, FU=follow-up, LGE=lategadolinium enhancement (Hirsch et al., 19 reprinted with permission of John Wiley & Sons, Inc.).
The HEBE trial in perspective
Left ventricular recovery following AMI in patients is a multifactorial process involving molecular, cellular, and physiological responses that balance debris removal and matrix repair. The importance of the inflammatory response in this process has been recognised for years and extensively studied. Presumably, the fate of the infarcted area as well as the viable myocardium surrounding the infarcted area is dependant on the effectiveness of the inflammatory response following the acute phase of AMI (figure 2). BMMCs play an important role in this response; however, their exact mechanisms of action are poorly understood. The results from a meta-analysis, comprising five randomised controlled trials, showed a beneficial effect of BMMC therapy on LVEF after AMI (2.3%; 95% CI: 0.8 to 3.8%).19 One should take into consideration that the increase in LVEF was on top of primary PCI and current state-of-the-art medical therapy. However, whether this beneficial effect is of clinical significance and which patient group benefits the most from BMMC therapy remains unclear. Post hoc analysis of the REPAIR-AMI trial suggested that BMMC therapy was most effective in patients with markedly depressed LVEF, indicating that the beneficial effects of bone marrow cell therapy may be confined to only a specific group of patients with AMI.12 Data from large, randomised controlled trials such as the HEBE trial are important to further determine the short- and long-term clinical effects of BMMC therapy after AMI and to evaluate its clinical significance. Furthermore, there are uncertainties about the timing of cell infusion, cell dosage, optimal cell type, and information about homing and survival of the transplanted BMMCs is still incomplete. There is no doubt that studying these issues will be best done in experimental models. However, an experimental model mimicking an AMI in the elderly patient does not exist. This may explain why attempts to translate experimental findings into clinical practice in the past have, in general, been disappointing. Therefore, a better understanding of the role of BMMCs in patients with AMI is necessary to improve treatment strategies. Interestingly, in the clinical trials, patients with AMI treated with BMMC therapy exhibit a marked variable response in left ventricular recovery (see also figure 1).12,14,19 Hence, comparative analysis of the data and material obtained from patients from the HEBE trial, showing either enhanced ventricular recovery or ventricular remodeling, may provide important insights into the contribution of BMMCs to natural myocardial recovery as well as the response to cell therapy. By validating the results obtained from the clinical trials in experimental models and vice versa, many unresolved issues concerning BMMC therapy may be clarified. This may significantly contribute to the development of improved cell-based therapies, aiming at optimising post-infarct recovery and preventing heart failure.
The final results of the HEBE trial were recently presented at the AHA Meeting 2008, in New Orleans, USA. The results of the HEBE show that intracoronary infusion of mononuclear cells from the peripheral blood or bone marrow, several days after primary PCI, does not improve global or regional left ventricular systolic function. A post-hoc analysis demonstrates that patients with an initially dilated left ventricle benefit from this mode of therapy as it prevents further dilatation of the left ventricle. This finding indicates that both peripheral and bone marrow mononuclear cells exert a beneficial effect on left ventricular remodelling following an acute myocardial infarction.
Acknowledgments
The HEBE study was initiated by Interuniversity Cardiology Institute of the Netherlands with support from the Netherlands Heart Foundation. We would like to thank all HEBE investigators.
References
- 1.Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation 2002;106:2351-7. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990;81:1161-72. [DOI] [PubMed] [Google Scholar]
- 3.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 2005;105:199-206. [DOI] [PubMed] [Google Scholar]
- 4.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res 2004;95:1191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol 2008;28:208-16. [DOI] [PubMed] [Google Scholar]
- 6.van den Bos EJ, van der Giessen WJ, Duncker DJ. Cell transplantation for cardiac regeneration: where do we stand? Neth Heart J 2008;16:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5. [DOI] [PubMed] [Google Scholar]
- 8.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med 2002;346:5-15. [DOI] [PubMed] [Google Scholar]
- 9.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol 2002;22:1804-10. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009-17. [DOI] [PubMed] [Google Scholar]
- 11.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breiden-bach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004;364:141-8. [DOI] [PubMed] [Google Scholar]
- 12.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210-21. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J 2006;27:2775-83. [DOI] [PubMed] [Google Scholar]
- 14.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J 2005;39:150-8. [DOI] [PubMed] [Google Scholar]
- 15.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J 2007;28:766-72. [DOI] [PubMed] [Google Scholar]
- 16.van Beem RT, Hirsch A, Lommerse IM, Zwaginga JJ, Noort WA, Biemond BJ, et al. Recovery and functional activity of mononuclear bone marrow and peripheral blood cells after different cell isolation protocols used in clinical trials for cell therapy after acute myocardial infarction. Eurointervention 2008;4:133-8. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch A, Nijveldt R, van der Vleuten PA, Biemond BJ, Doevendans PA, van Rossum AC et al. Intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells after primary percutaneous coronary intervention: rationale and design of the HEBE trial – a prospective, multicenter, randomized trial. Am Heart J 2006;152:434-41. [DOI] [PubMed] [Google Scholar]
- 18.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol 2002;22:1804-10. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch A, Nijveldt R, van der Vleuten PA, Tio RA, van der Giessen WJ, Marques KM, et al. Intracoronary infusion of autologous mononuclear bone marrow cells in patients with acute myocardial infarction treated with primary PCI: Pilot study of the multicenter HEBE trial. Catheter Cardio-vasc Interv 2008;71:273-81. [DOI] [PubMed] [Google Scholar]