Summary
Advances in molecular biology have provided tools for studying the epigenetic factors which modulate gene expression. DNA methylation is an epigenetic modification which can have sustained effects on transcription and is associated with long-term gene silencing. In this review, we focus on the regulation of estrogen receptor alpha (ERα) expression by hormonal and environmental cues, the consequences of these cues for female maternal and sexual behavior and recent studies which explore the role of DNA methylation in mediating these developmental effects, with particular focus on the mediating role of maternal care. The methylation status of ERα has implications for reproductive behavior, cancer susceptibility and recovery from ischemic injury suggesting an epigenetic basis for risk and resilience across the life span.
Introduction
The coordination of endocrine signals is essential to successful reproduction, particularly amongst mammals in which there is extensive prenatal and postnatal interaction between mothers and infants. During late gestation, circulating levels of estrogen increase and are essential for the up-regulation of peptide receptors involved in parturition, lactation and maternal behavior. Thus sensitivity to estrogen is critical to the change in behavior that promotes growth and survival of offspring through maternal investment. At a cellular and molecular level, estrogen is known to act through two distinct pathways: 1) through intracellular signaling following activation of membrane bound estrogen receptors and 2) through more classical genomic routes in which estrogen binds to nuclear estrogen receptors leading to transcriptional activation. Consequently, levels of estrogen receptor will determine the sensitivity to this hormone and ultimately regulate the efficiency of estrogen-mediated signaling and the biological and behavioral outcomes associated with estrogen. Recent evidence suggests that there are pervasive effects of the environment on the expression of estrogen receptors with implications for health and reproductive behavior. In particular, the alpha isoform of the estrogen receptor (ERα) has been found to be dynamically altered through epigenetic modification in response to physiological and behavioral cues. In this review, we will discuss emerging evidence for the role of environmental signals in regulating ERα, the role of DNA methylation in mediating these effects and the implications of these interactions between gene and environment on reproduction within and across generations.
ERα and Female Reproductive Behavior
Estrogen receptors belong to the nuclear hormone receptor family and dimerize in response to ligand binding to form a complex which promotes transcriptional activation of genes containing estrogen response elements (EREs). Though there are also non-genomic pathways of estrogen action involving membrane bound receptor activation and intracellular signaling with significant implications for physiology and behavior [1,2], the induction of transcription through activation of the two nuclear estrogen receptor isoforms, ERα and ERβ, are considered the classic route of estrogen effects [3]. Though both ER isoforms are expressed within the brain and have similar DNA binding domains [3,4], ERα and ERβ differ in ligand affinity and in the conformational changes that occur as a function of ligand binding [5]. Consequently, ERα has a greater affinity for estrogen and activation of this receptor isoform is associated with comparatively higher levels of transcriptional activity.
Pharmacological and genetic manipulations have been used to illustrate the role of ERα in the reproductive behavior of both male and female rodents. High levels of ERα expression are found in the hypothalamus, with particularly elevated expression within the medial preoptic area (MPOA), as well as the amygdala and ventral medial hypothalamus (VMH) [4]. The MPOA is critical for male sexual behavior [6] and female maternal behavior [7] whereas the VMH has been found to regulate female sexual receptivity [8]. Site-specific administration of the estrogen receptor antagonist 4-hydroxytamoxifen in the MPOA disrupts the onset of maternal responsivity amongst post-parturient females whereas administration of this antiestrogen into the VMH blocks the occurrence of the postpartum estrus [9]. ERα continues to influence maternal behavior during the postpartum period as indicated by c-fos activation in ERα positive cells in lactating female rats [10]. Targeted disruption of the ERα gene has been found to dramatically reduce the occurrence of lordosis and lead to increased rates of rejection of male attempts to initiate copulation [11]. These ERα knockout females also show elevated levels of infanticide and reduced motivation to retrieve pups indicating a broad spectrum of reproductive impairment. These behavioral deficits may be the consequence of abnormal development and regulation of oxytocin [12] and dopaminergic neuron signaling [13]. Mutation of ERα results in an elimination of estrogen-mediated up-regulation of oxytocin receptor binding in the several brain regions [12] and recent evidence suggests that striatal tyrosine hydoxylase levels are decreased in ERα-KO mice [13] which may account for the physiological, motivational and motor aspects of reproductive impairment in these females.
Early Environmental Regulation of ERα
One strategy for understanding the role of the environment in regulating ERα is to examine the consequences for ERα expression of developmental exposure to hormones, endocrine disruptors and peptides. Sexual dimorphism in ERα expression, with reduction in hypothalamic ERα in males compared to females, emerges developmentally [14] and is sustained into adulthood suggesting the organizational effects of circulating estrogens. Early treatment with elevated levels of this hormone have been found to decrease levels of ERα in the female brain and eliminate sex-differences in ERα expression [15]. The widespread use of xenoestrogens such as bisphenol A (BPA) in the manufacture of household plastics has lead to more thorough examination of the neuroendocrine and behavioral consequences of long-term exposure to the effects of synthetic estrogens. Neonatal treatment with high levels of BPA initially induces an increase in ERα with subsquent decreases in ERα expression within the MPOA [16]. The reproductive consequences of BPA-induced changes to hypothalamic estrogen receptors early in development include reduced sexual differentiation and significant reductions in the duration of maternal licking/grooming (LG) and frequency of nursing of pups during the postpartum period in adult females who were BPA-exposed as neonates [17,18]. Recent in vivo and in vitro studies have also shown that peripheral administration of oxytocin to female pups during the postnatal period can increase ERα in the VMH whereas administration of a selective oxytocin receptor antagonist can decreased ERα immunoreactivity in the MPOA [19,20]. These developmental effects on ERα are observed within the pre-weaning period and are sustained into adulthood. This may account for long-term oxytocin treatment effects including observed increases in adult sexual and social behavior [19,21].
Though direct targeting of ERα through pharmacological manipulation of the neuroendocrine system certainly has profound effects on behavior, similar regulatory influences can be achieved through modification of the early social environment. In rodents, natural variations in maternal care during the postpartum period are associated with long-term effects on offspring gene expression, physiology and behavior [22]. Comparison of the offspring of rat dams who engage in high vs. low levels of maternal LG indicates that exposure to low levels of this form of maternal care are associated with decreased hippocampal glucocorticoid receptor (GR) expression, increased hypothalamic-pituitary-adrenal response to stress and reduced exploration in a novel environment [23]. Female offspring of Low LG dams display high levels of sexual receptivity [24,25] yet engage in low levels of maternal LG toward their own offspring [26,27]. Individual differences in maternal LG are associated with variation in central oxytocin receptor density and ICV infusion of a selective oxytocin receptor antagonist decreases frequency of LG amongst High LG dams [28]. Female offspring of Low LG dams likewise have reduced central oxytocin receptor density and display reduced sensitivity to estrogen-induced up-regulation of neural activation and oxytocin receptor density within the hypothalamus [28–30]. This differential sensitivity is similar to what is observed amongst ERα KO females [12] and analysis of ERα expression in the MPOA as a function of postnatal maternal care confirms that the offspring of Low LG dams have reduced expression of this receptor isoform [29]. Cross-fostering of offspring from High LG to Low LG dams or from Low LG to High LG dams indicates that this difference in gene expression is associated with the quality of the postnatal environment [31]. Similar long-term effects on maternal behavior and ERα mRNA expression in the MPOA have been demonstrated in rat offspring who were cross-fostered between dams who were induced to be High or Low LG as a consequence of exposure to a predator odor [32]. Conversely, ERα mRNA in the anteroventral paraventricular nucleus of the hypothalamus is elevated amongst the female offspring of Low LG dams and these females are more sensitive to estrogen induced ERα activation within this region [33]. Thus, there are multiple hormonal and behavioral cues occurring early in development which exert site-specific regulatory influence on ERα with consequence for multiple aspects of reproduction.
Epigenetic Regulation of ERα Through DNA Methylation
The prolonged elevation in ERα levels that has been observed in response to early life experience suggests stable regulation of gene expression through epigenetic mechanisms. There are many modifications to chromatin structure which can alter transcriptional activity of the genome [34,35]. However, the most stable of these modifications is DNA methylation, in which a methyl group is attached by DNA methyltransferases to cytosine nucleotides within the DNA sequence. DNA methylation within the gene promotor generally prevents binding of transcription factors and RNA polymerase and is associated with gene silencing [36]. Methylation patterns are stable and heritable providing a pathway through which cellular differentiation can occur. Despite this stability, there is recent evidence for the dynamic regulation of gene promotor methylation in response to environmental condition, particularly those experiences occurring early in development. In the case of ERα, the differential expression of this receptor in response to variation in maternal care received in infancy has been found to be associated with methylation patterns within the ERα promotor. Comparison of the adult offspring of Low vs. High LG dams indicates elevated levels of ERα methylation at several of the CpG sites within the 1b promotor region in tissue taken from the MPOA of offspring of Low LG dams [31]. Consequently, there is less binding of transcription factors, such as Stat5b (signal transducer and activator of transcription 5b) to the ERα promotor [31]. Though the cellular/molecular pathway through which maternal care alters ERα methylation has yet to be elucidated, one potential route is through maternal up-regulation of transcription factors in the neonatal hypothalamus which promote ERα transcriptional activity and reduce the likelihood of epigenetic silencing. There is evidence for this activity/transcription factor-dependent pathway in the maternal regulation of GR expression, implicating serotonergic pathways and maternal up-regulation of NGFI-A (nerve growth factor-inducible protein-A) [37,38], however, this level of analysis has not yet been applied in the context of ERα. These long-term effects could also be indirectly mediated through LG stimulation of estrogen and oxytocin levels in the neonate which has been shown to regulate ERα expression in adulthood [15,20]. Future studies will focus on the possible routes through which these early behavioral and physiological events lead to modification of the epigenome.
The plasticity in ERα expression which can be achieved through DNA methylation has also been investigated in the context of cancer treatment and recovery following ischemic injury. The dysregulation of cell cycle that is characteristic of rapidly dividing cancer cells is associated with global hypomethylation and site specific hypermethylation, particularly of tumor repressor factors [35,39]. Elevated levels of DNA methylation of the ERα promotor are found in breast cancer cells leading to reduced ERα expression and a decreased efficiency of tamoxifen treatment [40], which works through the blocking of ERα. Advances in pharmacological targeting of the epigenome have lead to the development of several drugs which alter DNA methylation levels primarily through promotion of histone acetylation. Administration of histone deacetylase inhibitors, which increase histone acetylation and thereby decrease DNA methylation, in the treatment of cancer provides a novel approach to improving prognosis and in the case of ERα, has been found to increase estrogen sensitivity and the efficacy of tamoxifen treatment [41,42]. The origins of ERα expression and methylation abnormalities observed in cancer cells are yet unclear, however, there is recent interest in the potential role of environmental risk factors such as developmental exposure to xenoestogens which alter DNA methylation and is associated with increased cancer risk [43]. Interestingly, a recent report indicates that the epigenetic abnormalities associated with in utero BPA exposure can be reversed through maternal dietary supplementation with genistein, folic acid, choline and betaine [44] which serve as methyl donors within the DNA methylation process [45]. Though these studies are compelling, the site-specificity, gene specificity and developmental timing of these global treatments must be considered in evaluating the consequence of this therapeutic approach.
Changes in the expression of ERα occur across development and within the reproductive cycle. Though ERα levels are elevated in the hypothalamus in both early development and in adulthood, in the cortex there is a significant decrease in ERα in the adult brain [46]. However, ischemic injury results in a rapid increase in cortical ERα in female rodents and may serve to enhance neuroprotection following injury [47]. Estrogen has previously been shown to prevent cortical damage following an ischemic episode [48] and using ERα-KO mice, this effect has been shown to be ERα-dependent [49]. Recent evidence suggests that DNA methylation of the ERα promotor is decreased in rodent cortical tissue of females but not males following ischemic injury [50]. Thus, pre-ischemic and post-ischemic factors that modulate the DNA methylation pathway and the regulation of ERα may determine functional recovery following injury.
Conclusion
The expression of ERα has functional implications for reproductive behavior and health and can be regulated through multiple hormonal and environmental pathways occurring developmentally and in adulthood (Figure 1). Recent evidence suggests that variation in DNA methylation of the ERα promotor can be induced by the quality of the early maternal environment with long-term consequences for the maternal behavior of female offspring. The behavioral transmission of these epigenetic maternal effects from mother to offspring [26,31] suggests that any environmental condition which can alter ERα expression within the hypothalamus may have implications for the reproductive behavior of subsequent generations. Thus, pharmacological manipulations that target DNA methylation globally and have been shown to modify ERα gene expression could induce transgenerational effects on health and behavior. Our current understanding of the epigenetic regulation of gene expression and the implications of this regulation for individual differences in physiology and behavior has advanced rapidly through use of molecular and cellular approaches. In future, these studies may provide further insight into the biological basis of the interaction between genes and environment and the developmental origins of long-term reproductive outcomes.
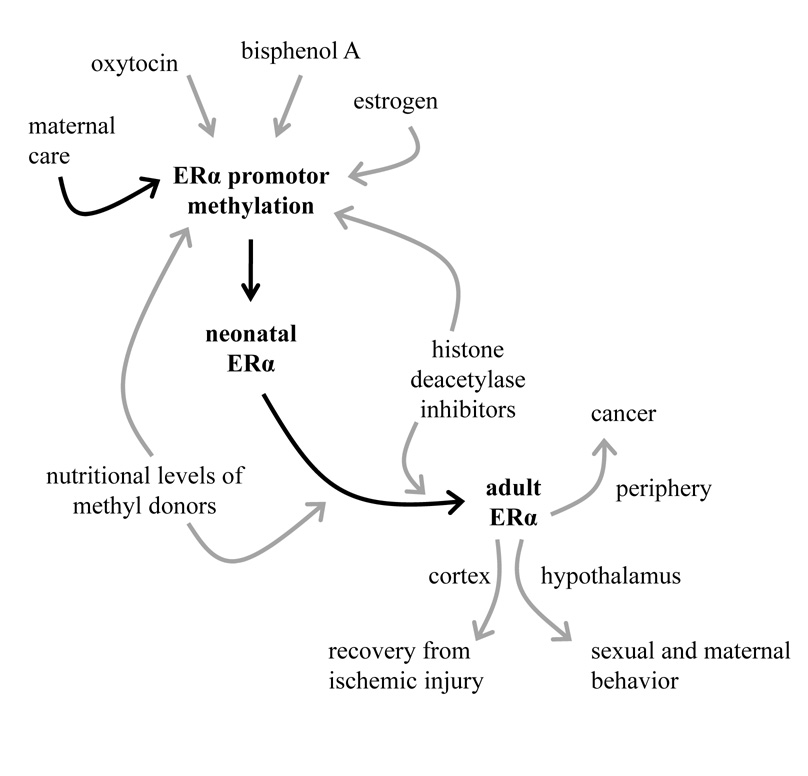
Figure 1.
Potential regulatory pathways of early environment influence on adult ERα expression. Maternal care has been demonstrated to alter site-specific ERα promotor methylation whereas neonatal oxytocin, bisphenol A and estrogen treatment have been demonstrated to exert long-term influence on ERα expression with the role of DNA methylation yet to be elucidated. Gene expression in infancy and adulthood can be modified epigenetically through dietary intake of methyl donors such as folic acid and genistein or through administration of histone deacetylase inhibitors which promote reduced DNA methylation. Consequently, adult ERα expression has site specific effects on health and behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: Evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 4.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 6.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan-Cato LM, Calizo LH, Daniels D. The synaptic organization of VMH neurons that mediate the effects of estrogen on sexual behavior. Horm Behav. 2001;40:178–182. doi: 10.1006/hbeh.2001.1679. [DOI] [PubMed] [Google Scholar]
- 9.Ahdieh HB, Mayer AD, Rosenblatt JS. Effects of brain antiestrogen implants on maternal behavior and on postpartum estrus in pregnant rats. Neuroendocrinology. 1987;46:522–531. doi: 10.1159/000124875. [DOI] [PubMed] [Google Scholar]
- 10.Lonstein JS, Greco B, De Vries GJ, Stern JM, Blaustein JD. Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology. 2000;72:91–101. doi: 10.1159/000054576. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 12.Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9:933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- 13. Kuppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-alpha knockout (ERKO) mice. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.03.007. Using ERα knockout mice, the authors demonstrate the influence of this receptor for the development of dopaminergic systems in males and females which may have implications for the deficits in social and cognitive functioning previously observed in ERα null mice.
- 14.Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sex differences in the development of estrogen receptors in the rat brain. Horm Behav. 1994;28:483–491. doi: 10.1006/hbeh.1994.1046. [DOI] [PubMed] [Google Scholar]
- 15.DonCarlos LL, McAbee M, Ramer-Quinn DS, Stancik DM. Estrogen receptor mRNA levels in the preoptic area of neonatal rats are responsive to hormone manipulation. Brain Res Dev Brain Res. 1995;84:253–260. doi: 10.1016/0165-3806(94)00179-4. [DOI] [PubMed] [Google Scholar]
- 16. Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5'-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. Examines the developmental effects of BPA exposure on ERα expression and promoter methylation. Though there is a significant effect on hypothalamic ERα gene expression, the authors do not find an association with DNA methylation.
- 17.Della Seta D, Minder I, Dessi-Fulgheri F, Farabollini F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull. 2005;65:255–260. doi: 10.1016/j.brainresbull.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110 Suppl 3:415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Carter CS, Cushing BS. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience. 2006;137:157–164. doi: 10.1016/j.neuroscience.2005.08.065. Using a model in which monogamous prairie voles are treated developmentally with oxytocin or an oxytocin receptor antagonist, the authors demonstrate the positive association between oxytocin levels in infancy and adult ERα expression.
- 21.Cushing BS, Levine K, Cushing NL. Neonatal manipulation of oxytocin influences female reproductive behavior and success. Horm Behav. 2005;47:22–28. doi: 10.1016/j.yhbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 24.Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm Behav. 2008;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Uriarte N, Breigeiron MK, Benetti F, Rosa XF, Lucion AB. Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev Psychobiol. 2007;49:451–462. doi: 10.1002/dev.20241. [DOI] [PubMed] [Google Scholar]
- 26.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 27.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 28.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 30.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 31. Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. The first demonstration of maternal regulation of DNA methylation of the ERα promoter in female offspring receiving high vs. low levels of care with implications for the transmission of maternal behavior across generations. The authors include cross-fostering data to illustrate the non-genomic basis of this maternal effect.
- 32. McLeod J, Sinal CJ, Perrot-Sinal TS. Evidence for non-genomic transmission of ecological information via maternal behavior in female rats. Genes Brain Behav. 2007;6:19–29. doi: 10.1111/j.1601-183X.2006.00214.x. The authors show the effects of predator odor on maternal care and the transmission of these effects across generations involving differential expression of ERα in cross-fostered female offspring.
- 33. Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE. 2008;3:e2210. doi: 10.1371/journal.pone.0002210. The authors explore the role of maternal care in regulating offspring sexual behavior and demonstrate the role of site specific changes in ERα in mediating this effect. Taken together with [31] illustrates trade-offs in reproduction as a function of early life experience.
- 34.Mellor J. Dynamic nucleosomes and gene transcription. Trends Genet. 2006;22:320–329. doi: 10.1016/j.tig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Strathdee G, Brown R. Aberrant DNA methylation in cancer: potential clinical interventions. Expert Rev Mol Med. 2002;4:1–17. doi: 10.1017/S1462399402004222. [DOI] [PubMed] [Google Scholar]
- 36.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 38. Weaver IC, D'Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. Illustrates the role of NFGI-A in mediating the effects of maternal care on DNA methylation within the GR promotor and provides insight into cellular/molecular pathways through which maternal care exerts epigenetic effects.
- 39.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 40.Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006;11:1–8. doi: 10.1634/theoncologist.11-1-1. [DOI] [PubMed] [Google Scholar]
- 41.Fan J, Yin WJ, Lu JS, Wang L, Wu J, Wu FY, Di GH, Shen ZZ, Shao ZM. ERalpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134:883–890. doi: 10.1007/s00432-008-0354-x. [DOI] [PubMed] [Google Scholar]
- 42.Jang ER, Lim SJ, Lee ES, Jeong G, Kim TY, Bang YJ, Lee JS. The histone deacetylase inhibitor trichostatin A sensitizes estrogen receptor alpha-negative breast cancer cells to tamoxifen. Oncogene. 2004;23:1724–1736. doi: 10.1038/sj.onc.1207315. [DOI] [PubMed] [Google Scholar]
- 43.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 44. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. Using agouti mice, the authors show the effects of DNA methylation of prenatal exposure to BPA and the amelioration of this effect through maternal dietary intake of methyl donors. These results demonstrate the plasticity of these epigenetic mechanisms in response to environmental cues.
- 45.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 46.Guo XZ, Su JD, Sun QW, Jiao BH. Expression of estrogen receptor (ER) -alpha and -beta transcripts in the neonatal and adult rat cerebral cortex, cerebellum, and olfactory bulb. Cell Res. 2001;11:321–324. doi: 10.1038/sj.cr.7290103. [DOI] [PubMed] [Google Scholar]
- 47.Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, et al. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 48.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Westberry JM, Prewitt AK, Wilson ME. Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience. 2008;152:982–989. doi: 10.1016/j.neuroscience.2008.01.048. The first paper to demonstrate changes in DNA methylation within the ERα promoter following ischemic injury. Suggest that changes to the epigenetic status of ERα can promote neuroprotection.