Abstract
Response selection activates appropriate response representations to task relevant environmental stimuli. Research implicates dorsolateral prefrontal cortex (dlPFC) for this process. On the other hand, studies of semantic selection, which activates verbal responses based on the semantic requirements of a task, implicate ventrolateral PFC (vlPFC). Despite this apparent dissociation, the neurocognitive distinction between response and semantic selection is controversial. The current functional MRI study attempts to resolve this controversy by investigating verbal response and semantic selection in the same participants. Participants responded vocally with a word to a visually presented noun, either from a memorized list of paired associates (response selection task), or by generating a semantically related verb (semantic selection task). We found a dissociation in left lateral PFC. Activation increased significantly in dlPFC with response selection difficulty, but not semantic selection difficulty. Conversely, semantic, but not response, selection difficulty increased activity significantly in vlPFC. Activity in left parietal cortex, on the other hand, was affected by difficulty increases in both selection tasks. These results suggest that response and semantic selection may be distinct cognitive processes mediated by different regions of lateral PFC; but both of these selection processes rely on cognitive mechanisms mediated by parietal cortex.
We are constantly surrounded by a large variety of sensory information in our environment. However, not all of the available information is relevant to our current tasks and goals. Therefore we must selectively process stimuli that are important for our current situation and ignore the rest. Likewise, in order to behave appropriately, we must select task-relevant responses from the set of possible responses. Selection is thus a fundamentally important, and highly adaptive, function of our information processing system.
Various kinds of selection processes are carried out in the brain – from early perceptual selection mechanisms in primary and secondary visual cortices (e.g., Kastner & Ungerleider, 2000) to the selection of appropriate memory and response representations mediated by prefrontal, parietal, and other cortical regions. It is the selection carried out by regions in association cortex that is the focus of the research described here. Response selection, for example, retrieves a representation for an appropriate motor response among competing alternatives (Kornblum, Hasbroucq, & Osman, 1990). Semantic selection, on the other hand, as investigated here, activates the representation of a word based on its semantic properties (Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997). Theoretical constructs of selection (e.g., Pashler, 1994) as well as the conceptual similarity of these selection processes (i.e., retrieval of task relevant information) suggests that there may be a unitary selection process underlying both types of selection.
Research on selection suggests that prefrontal cortex (PFC) plays a key role. Selection may be an example of a more general function of the PFC (i.e., the implementation of cognitive control). It has been proposed that PFC exerts top-down control on posterior brain regions (Fuster, 2000; Kan & Thompson-Schill, 2004; Miller & Cohen, 2001; Petrides, 2000; Schneider & Chein, 2003). According to these accounts, PFC (especially lateral PFC) is at the top of a processing hierarchy and regulates activity in subcortical and posterior cortical brain regions. Selection likely is one instance of this top-down influence. Consistent with this idea, posterior regions in parietal and temporal cortex have been found to be co-activated with frontal cortex during selection tasks (Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002; Schumacher, Cole, & D'Esposito, 2007; Schumacher, Hendricks, & D'Esposito, 2005; Thompson-Schill et al., 1997).
Consistent with the view that selection is a form of cognitive control, many studies of selection find activity in lateral PFC, as well as posterior cortical regions. However it is not clear if selection relies on a unitary or on separable neural mechanisms. According to the selection hypothesis proposed by Thompson-Schill and colleagues (1997) and Rowe and colleagues (2000) selection may be a unitary function implemented in lateral PFC. Other authors suggest that the neural implementation of selection might differ between response and non-response selection (Zhang, Feng, Fox, Gao, & Tan, 2004). Furthermore, studies investigating different kinds of selection processes (e.g. spatial response selection, numeric response selection, semantic selection) report the recruitment of distinct brain regions (Fletcher & Henson, 2001; Hazeltine, Bunge, Scanlon, & Gabrieli, 2003; Schumacher, Elston, & D'Esposito, 2003; Thompson-Schill et al., 1997).
Thus, the neuroimaging evidence for a unitary selection process is equivocal, with some data supporting a general selection mechanism, and other data reporting separate neural systems for selection of different types of information. Behavioral data regarding response selection are similarly ambiguous. Some behavioral data suggest that response selection may be a unitary process (for a review see Pashler, 1994), whereas other data suggest the existence of multiple selection processes (Hazeltine, Teague, & Ivry, 2002; Meyer & Kieras, 1997; Schumacher et al., 1999; Schumacher et al., 2001).
The present study investigates the neurocognitive overlap between semantic and response selection, and more specifically, whether there are regional differences in the PFC regions mediating these mechanisms. Several authors report that semantic selection mainly activates ventrolateral PFC (vlPFC; Crosson et al., 2001; Thompson-Schill et al., 1997; Tremblay & Gracco, 2006), whereas studies of response selection mainly activate dorsolateral PFC (dlPFC; Schumacher & D'Esposito, 2002; Schumacher et al., 2003). Yet, some studies of semantic selection (Buckner, Raichle, & Petersen, 1995; Thompson-Schill et al., 1997; Thompson-Schill et al., 1998) report dlPFC activation as well. And finally, some studies of response selection have found vlPFC activation (Fletcher & Henson, 2001; Rowe & Passingham, 2001). Thus, current research suggests that response selection and semantic selection may involve partially overlapping brain regions, but there is also evidence that these processes may be cognitively and neurally distinct. The goal of the present study is to directly compare response and semantic selection in the same participants and with the same stimulus and response modalities.
In this study, we used visual-vocal selection tasks with verbal material to match the stimulus and response modalities between the conditions. Participants performed a paired-associate task, which is a typical response selection task; and a word-generation task, which is a typical semantic selection task (c.f., Thompson-Schill et al., 1997). During the Thompson-Schill and colleagues experiment, participants were presented with a noun and were instructed to covertly generate a semantically related verb (e.g. scissors-cut). In the present study, participants made overt verbal responses. Thus we are able to measure response accuracy and response times (RTs), which could not be done by Thompson-Schill and colleagues. Response selection was tested with a design similar to that of previous response selection studies (Schumacher et al., 2003). Schumacher and colleagues had participants learn a set of number-response pairs. During testing, participants were presented with the number and had to retrieve the associated manual response. In the present study, both the stimuli and responses used verbal material.
The direct comparison of semantic selection and verbal response selection in the present study allows us to investigate the hypothesis that these two types of selection rely on different lateral PFC regions. Additionally, it allows us to examine the suggestion made by Thompson-Schill and colleagues, (Snyder, Feigenson, & Thompson-Schill, 2007; Thompson-Schill et al., 1997) that vlPFC mediates selection at a more general level than for semantic material only. Finally, comparing the activation in the verbal response selection task with activation patterns from previous response selection studies using different stimulus and response modalities, but similar procedures, will allow us to investigate the modality and material specific nature of response selection.
Importantly, we parametrically manipulated both conditions (i.e., the difficulty of selection varied across several levels). This has several methodological advantages. It reduces our reliance on the critical assumption of pure insertion, allows us to more clearly differentiate between response selection, motor planning, and motor production, and allows us to distinguish between selection-specific and general task-related activity. This approach has been employed successfully in a number of previous studies (e.g., Braver et al., 1997; Jonides et al., 1997; Manoach, 2003; Schumacher et al., 2003).
Methods
Participants
Fourteen right-handed volunteers from the University of California, Berkeley community (6 male, 8 female; age range 19–26 years; mean age 22 years) participated in our study. All participants gave their informed consent and were paid $8 per hour for their participation. They were native English speakers with no history of neurological disorders or medication. Three participants were excluded from the analysis due to excessive motion (viz., more than 3mm during the between-run intervals). Thus, the final sample contained 11 participants (5 male, 6 female; mean age 21).
Behavioral Task Design and Stimuli
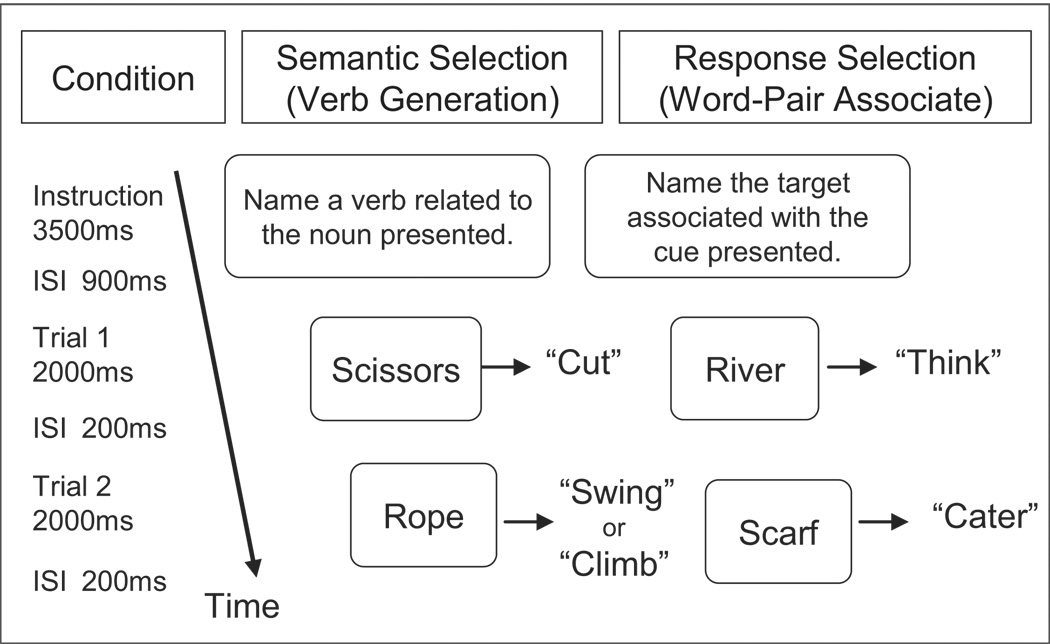
Response selection and semantic selection were parametrically manipulated in separate blocks of trials. For all blocks, participants made verbal responses to centrally presented stimulus words. The words were presented in white on a black background. Participants viewed them through a mirror mounted on the radiofrequency (RF) head coil. The stimuli subtended roughly 4–6° visual angle. A schematic of the experimental design is shown in Figure 1.
Figure 1.
Schematic of the experimental design. Participants performed a semantic selection task in which they responded to a visually presented noun with an associated verb and a response selection task in which they responded to a visually presented cue noun with a target word associated with it during a previous study session. Selection difficulty varied across blocks (see Method). Participants also performed a fixation control task in which they focused on a fixation stimulus throughout the block, which is not depicted in the figure.
Semantic Selection Task
Semantic selection was manipulated with the verb generation procedure used by Thompson-Schill and colleagues (1997), in which participants vocally responded with a semantically related verb to a visually presented noun. We used the 96 concrete nouns (word length range [3–8], median = 4; Kucera-Francis frequency range [1–591], median frequency = 32]) used by Thompson-Schill and colleagues.
There were two levels of difficulty for this task. In the low selection demand condition, nouns were highly associated with one or two related verbs (e.g. knife – cut; associative strength range [5–50], median = 13.34; mean Kucera-Francis frequency = 51.38; length = 3-8). In the high selection demand condition, nouns had no highly associated verb (associative strength range [1–3], median = 2.00; mean Kucera-Francis frequency = 69.21; length = 3–7). The difference between the associative strengths of the word groups was significant: t (94) = 13.85, p < 0.001.
Response Selection Task
The verbal response selection condition was similar to the semantic selection condition. Again, participants vocalized a verb in response to a presented noun. However, for this condition we used a paired-associate memory retrieval task. On the day prior to scanning, participants studied 8 word-pairs consisting of a noun and a verb each (e.g. dove-brew, scarf-cater). During the scanning session, the nouns from this list were presented one at a time and participants had to vocally respond with the associated verb.
Each of the word pairs were semantically unrelated (unassociated according to the Edinburgh Associative Thesaurus; mean Kucera-Francis frequency = 69.51; length = 4-7). Verbal response selection difficulty was varied parametrically by manipulating stimulus set size. Eight word-pairs were used in the most difficult condition, four pairs in the intermediate condition, and two in the easiest. For the four-choice and two-choice conditions, the nouns were repeated equally so that each block contained eight nouns. Both the increasing number of stimulus-response (S-R) rules active across blocks and the decrease in the priming of upcoming S-R rules likely affected response-selection difficulty across blocks. At the beginning of each block, participants were informed which words would be tested. Within the experimental blocks, the words were presented in randomized order. This design is conceptually similar to the way response selection was manipulated by Schumacher and colleagues (2003).
The experiment also included a fixation control condition in which participants fixated on a centrally presented white cross for about 8 seconds. Two additional conditions were included but will not be discussed here.
Behavioral procedure
One day prior to the scanning session, participants studied the list of the 8 word-pairs. First, participants viewed the word-pairs on a sheet of paper for several minutes. Then, they were trained to a criterion of 100% correct. Each noun was randomly presented one at a time on a computer monitor. Participants were required to say the associated verb aloud as quickly as possible. They were given feedback after every block about their accuracy and mean RT.
Before entering the scanner, participants were verbally instructed about the tasks they would perform. They completed 6 fMRI runs. During each run, each condition was tested in a block of 8 trials and appeared twice in random order so that participants saw 96 trials of each condition across the 6 runs. Each block began with a 3500 ms instruction period, in which the upcoming task and word lists were presented. The stimulus word followed a 900ms inter-stimulus interval (ISI). Each stimulus word appeared for 2000 ms. There was a 200 ms ISI between each trial (see Figure 1). A blocked design was used for this study in order minimize the effects of motion-related artifacts due to overt speech (c.f., Birn, Cox, & Bandettini, 2004) and to increase statistical power because PFC activity is known to be more variable and less significant than sensory regions (D'Esposito, Zarahn, & Aguirre, 1999).
fMRI procedure
Functional MRI scans were collected using a 4.0 Tesla Varian Inova scanner equipped with a fast gradient system for echoplanar imaging. A standard RF head coil was used with foam padding to restrict head motion comfortably. A two-shot gradient echo, echoplanar sequence (TR = 2200 ms, TE = 28 ms, matrix size = 64 × 64, FOV = 22.4 cm) was used to acquire data sensitive to the blood oxygen level (BOLD) dependent signal. Each functional volume contained 20-3.5 mm axial slices with a 0.5 mm gap between slices. Each experimental run was preceded by 5 seconds of dummy gradient RF pulses to achieve a steady state of tissue magnetization. Each run lasted just over 8 minutes and consisted of 222 brain volumes. Two high-resolution structural T1-weighted scans were also acquired. The first collected 20 axial slices in the same plane as the echoplanar images (TR = 200 ms, TE = 5 ms, matrix size = 256 × 256, FOV = 22.4 cm). The second was a 3D MPFLASH scan (TR = 9 ms, TE = 4.8 ms, TI = 300 ms). Vocal responses were collected in the scanner using a MR compatible microphone attached to a modified SCUBA mouthpiece. The mouthpiece was used to filter out the gradient noise (c.f., Stelzel, Schumacher, Schubert, & D'Esposito, 2006).
fMRI Data processing and analysis
During reconstruction, two images were created for each scan by linearly interpolating each adjacent scan. All additional data pre-processing and analysis was conducted using BrainVoyager QX (BrainInnovation, Maastricht, the Netherlands). Pre-processing included a slice scan time correction, 3-D motion correction (Friston, Frith, Turner, & Frackowiak, 1995), and high-pass temporal filtering (frequencies lower than 6 cycles per run were removed).
Data were analyzed with a modified general linear model (Worsley & Friston, 1995). For each participant, we created a design matrix with covariates for each level of each of the six behavioral conditions (eight-choice, four-choice, two-choice, high-selection demand, low-selection demand, and fixation control). These covariates were convolved with an idealized model of the hemodynamic response function. Seven runs (out of 66) were omitted from the final analysis due to motion (greater than 3mm). To account for head motion in the remaining runs, the six 3-D motion parameters were also included into the GLM as nuisance variables.
Region-of-Interest (ROI) analysis
All participants produced task-related activity (identified by contrasting combined task conditions [high and low semantic selection, and hard, medium and easy response selection] with the fixation control condition) in frontal, parietal, temporal and occipital cortical regions.
To investigate how activity changed across conditions, we used the task-related activity to create ROIs for brain regions of theoretical interest. We focused our analyses on ROIs from left dlPFC, left vlPFC, and left inferior parietal cortex (iPC)1 because these regions produced consistent activity across our participants and have been implicated in previous studies of selection (Schumacher & D'Esposito, 2002; Schumacher et al., 2003; Thompson-Schill et al., 1997). Using BrainVoyager software, ROIs were functionally defined separately for each participant in each of these regions by identifying clusters of voxels with a peak activity with a t-value corresponding to p < 0.005, uncorrected. The exact location and size of each ROI varied somewhat within each anatomical region across participants. The mean ROI center (and corresponding Brodmann area) and size across participants is shown in Table 1. To investigate laterality effects in dlPFC, an ROI was created for right dlPFC in every participant homologous to the left dlPFC ROI.
Table 1.
Location and statistics of selected Regions-of-Interest
| Talairach coordinates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Brodmann Area | X | Y | Z | Region-of-Interest (mean # voxels/standard deviation) | Selection Task F(1,10) = | Task Difficulty F(1,10) = | Interaction F(1,10) = | Response Selection 8 vs. 2 (one-tailed) t(10) = | Semantic Selection Hi vs. Lo (one-tailed) t(10) = |
| Left Dorsolateral Prefrontal Cortex |
9/46 | −42 | 30 | 23 | 251 (418) | 1.66, p=.23 | 7.28, p<.05 | 1.04, p=.34 | 2.10, p<.05 | 1.08, p=.15 |
| Left Ventrolateral Prefrontal Cortex |
45/47 | −48 | 23 | 2 | 57 (65) | .24, p=.63 | 3.06, p=.11 | 2.24, p=.17 | .001, p=.50 | 2.19, p<.05 |
| Inferior Parietal Cortex |
7/40 | −44 | −42 | 41 | 550 (557) | 06, p=.82 | 8.59, p<.05 | .27, p=.62 | 2.52, p<.05 | 2.00, p<.05 |
For each participant and ROI, mean β-values were extracted separately for each experimental condition relative to the fixation control condition. This led to five activation values for each participant. These β-values served as data for subsequent analyses.
Investigation of overt speech induced artifacts in BOLD signal
Several studies have reported that overt speech and other movements of the mouth or near the mouth may affect the in BOLD signal – especially in the operculum and insula regions, which are near the vlPFC (e.g., Birn, Bandettini, Cox, Jesmanowicz, & Shaker, 1998; Birn et al.,2004; Kemeny, Ye, Birn, & Braun, 2005; Yetkin et al., 1996). Therefore, it is important to consider the quality of the BOLD signal in the present data. Several factors may ameliorate a concern with the quality of the data produced here. First, the negative effect of overt speech on the BOLD signal is more pronounced with continuous speech (e.g., Kemeny et al., 2005) than with the short bursts (mean duration less than 700 ms) of discrete speech used here. In fact, the procedure used in the current study has been used previously to successfully record vocal responses and BOLD signal (Stelzel et al., 2006; see also Barch et al., 1999). Second, as stated previously, head motion was not excessive (less than 3 mm) in most participants overall and in none of the participants used in the analyses reported here.
However, overt speech may produce susceptibility artifacts (e.g., signal drop out) in the BOLD signal even in the absence of excessive head motion (Birn et al., 1998). Yet, each participant in this study produced activity in the ventrolateral, dorsolateral, and inferior parietal regions. These factors lead us to believe that our results are not overly contaminated by speech related artifacts; and give us confidence that comparisons of activity across the specific speech related conditions (e.g., eight-choice vs. two-choice and high selection vs. low) described below may provide valid insights into the nature of selection in lateral prefrontal and inferior parietal cortices.
Results
Behavioral Data
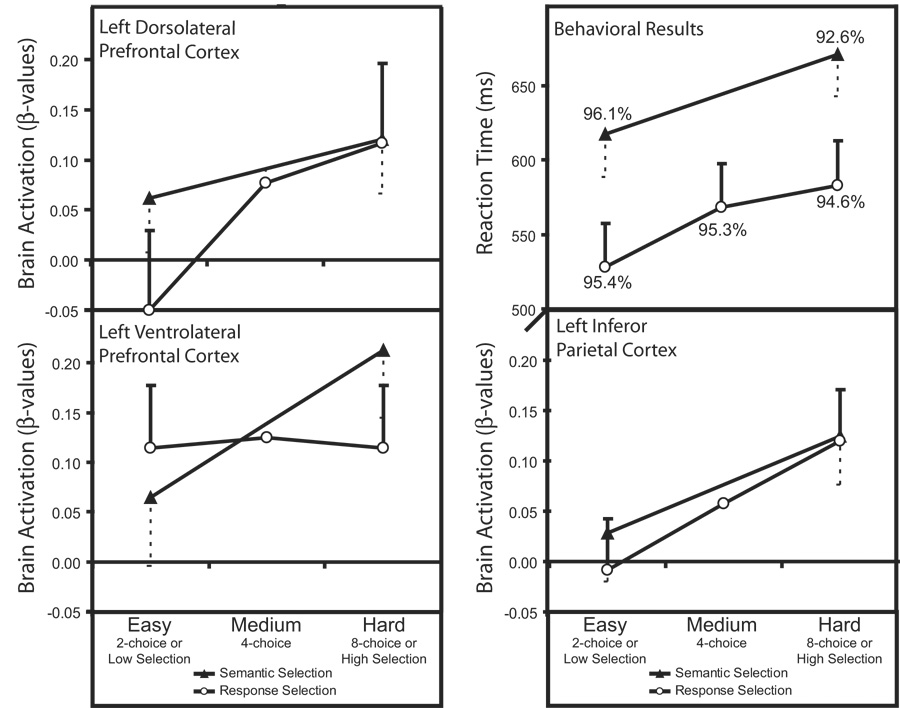
Reaction times and accuracies were measured for the vocal responses recorded during fMRI scanning. Vocal RTs less than 250 ms were excluded from subsequent analyses (6% of the responses) to avoid contamination of the data from trials where the response indicated nonverbal acoustic sounds (e.g., gradient noise, participants throat clearing, etc.) rather than participants’ vocalizations. Due to technical problems with the voice recording device, RT data were not measured for three participants. Figure 2 shows the mean RTs for correct trials and mean accuracies for the remaining participants. The correct RTs and accuracy data were analyzed separately for each selection task. For response selection, a repeated-measures analysis of variance (ANOVA) showed that mean RTs increased significantly with task difficulty (F[2,12] = 11.06, p < .005). Similarly, for semantic selection, mean RTs were longer for the high selection condition than the low selection conditions (t(6) = 3.37, p < .05). There was no significant effect on accuracy for either selection task: (F[2,18] = 1.91, p > .15 for response selection and t(9) = − 1.92, p > .25 for semantic selection). The trend across participants was for accuracy to decrease as selection difficulty increased, therefore there is no evidence that participants were trading speed for accuracy in this experiment.
Figure 2.
Mean reaction times, accuracies and β-values across difficulty levels for the response selection (two-choice, four-choice, and eight-choice) and semantic selection tasks (low and high selection). The standard errors are plotted for the comparison between the easy and hard conditions for each selection task.
fMRI Data
The mean β-values (representing brain activity) for each task condition relative to the fixation baseline are shown for each ROI in Figure 2. These β-values were analyzed separately for each ROI with repeated measures ANOVA using Selection task (response and semantic) and Difficulty (eight-choice/high and two-choice/low) as factors. These results are shown in Table 1. The ANOVA revealed significant effects of Difficulty on activity in left dlPFC and iPC, and a marginal effect of Difficulty in left vlPFC. Because of the specific hypotheses for activity in these ROIs, planned t-tests also compared eight-choice vs. two-choice and high selection vs. low in each ROI. Mean activity in the four-choice response selection condition fell within the two-and eight-choice conditions for every ROI. These data are shown in Figure 2, but were not included in the statistical analyses to match the comparisons across selection tasks.
As shown in Figure 2, the planned comparisons of the Difficulty effect within each selection task produced a dissociation between the tasks. Response selection task difficulty significantly affected activity in left dlPFC but produced no increase in activity in left vlPFC. Conversely, semantic selection difficulty significantly affected activity in left vlPFC and produced a nonsignificant increase in left dlPFC. Activity in iPC was significantly affected by increasing task difficulty in both selection tasks.
There was no significant effect of response selection difficulty on activity in right dlPFC (β-values: two-choice condition = 0.23, eight-choice condition = 0.09; t(10) = −1.89). The interaction between hemisphere and task difficulty on dlPFC activity was significant; F(1,10) = 7.70, p < .05.
Discussion
Research suggests that response selection is mediated by a network of brain regions including left dlPFC and left iPC (e.g., Schumacher et al., 2003); whereas semantic selection is mediated by a network including left vlPFC and left iPC (e.g., Thompson-Schill et al., 1997). This neural dissociation between these processes may suggest a cognitive dissociation as well. However, other research suggests that the regions mediating response and semantic selection may overlap (c.f., Fletcher & Henson, 2001). Because these selection processes have not been investigated in the same experiment, these inconsistent results may be due to differences in experimental design, materials, participants or a combination of these factors. The current study directly investigated the neurocognitive overlap between these two selection processes with a design that equated the visual stimulus and verbal response processing requirements across tasks.
We found a dissociation in lateral PFC. Brain activity in left dlPFC increased significantly with increases in response selection difficulty and insignificantly with semantic selection; whereas activity in left vlPFC increased significantly with increases in semantic selection task demand, but was unaffected by increases in response selection difficulty. This dissociation suggests that PFC may be functionally specialized for different types of selection. Activity in iPC, on the other hand, significantly increased with increases in both types of selection. This is consistent with previous reports implicating this region in both response and semantic selection (e.g. Schumacher et al., 2003; Thompson-Schill et al., 1997). It may reflect an increase in working memory and/or attentional load as the tasks get harder (c.f., Chein, Ravizza, & Fiez, 2003; Corbetta, Kincade, & Shulman, 2002). With the current design, it is difficult to specify the exact nature of this cognitive process; nevertheless these data suggest that neutrally distinct prefrontal mechanisms may mediate response and semantic selection processes by modulating a network of at least partially overlapping posterior brain regions (e.g., iPC).
This dissociation in activity between left dlPFC and vlPFC for different selection processes is consistent with previous reports in the literature suggesting dlPFC mediates response selection (Bunge et al., 2002; Crosson et al., 2001; Hazeltine et al., 2003; Jiang & Kanwisher, 2003; Rowe & Passingham, 2001; Rowe et al., 2000; Schumacher et al., 2007; Schumacher & D'Esposito, 2002; Schumacher et al., 2003) and vlPFC mediates semantic selection (Hester, D'Esposito, Cole, & Garavan, 2007; Thompson-Schill et al., 1997; Tremblay & Gracco, 2006). Thus, although the dissociation for the neural mechanisms for response and semantic selection suggested here has not been demonstrated before, the present pattern of results is consistent with existing theories of the neural mechanisms for selection.
Several authors have claimed that lateral PFC mediates a regulatory function by modulating representations and processing in posterior brain regions (Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005; Fuster, 2000; Kan & Thompson-Schill, 2004; Miller & Cohen, 2001; Petrides, 2000; Thompson-Schill, Bedny, & Goldberg, 2005). In the context of our current experiment, selection may be one instance of this regulatory function. Top-down selection signals may originate from distinct lateral prefrontal regions depending on selection type; but terminate in the same iPC regions. That is, during selection, memory contents maintained through iPC may be selectively activated in response to ones current goals or task demands. Importantly, this idea that lateral PFC fulfills a general control function does not necessarily imply a lack of functional segregation within PFC. Our results suggest that the specific nature of the selection task (response or semantic) recruits different lateral PFC regions.
Episodic retrieval mechanisms may be one possible cause for the differential involvement of lateral PFC regions in these tasks. Whereas the semantic selection task requires participants to select words based on semantic associations, the response selection task requires participants to recall the word episodically associated with its presented pair. As described by Dobbins and Wagner (2005), semantic elaboration and semantic selection typically activate vlPFC, whereas dlPFC is activated by general episodic retrieval tasks. Thus, one reason for the difference between the selection-related activity reported here might be that response selection is based on retrieval from episodic memory, whereas semantic selection follows semantic retrieval.
Dorsolateral PFC activity increased (although not significantly) with semantic selection task difficulty (see Figure 2). It is difficult to interpret this increase. It may suggest, as proposed by Fletcher and Henson (2001) that left dlPFC is involved in semantic selection; or that the semantic selection task used here also requires response selection. Alternatively, the non-significant activation increase in dlPFC during semantic selection may be spurious. Some evidence for this comes from a study of brain damaged patients by Thompson-Schill and colleagues (1998). They investigated semantic selection in patients with left vlPFC lesions; left frontal (including dlPFC but not vlPFC) lesions; and right frontal lesions. They found a semantic selection deficit only in the left vlPFC patients. Damage to regions outside left vlPFC, including dlPFC, did not affect semantic selection. This suggests, along with other studies implicating left vlPFC in semantic selection (e.g., Badre et al., 2005; Fletcher, Shallice, & Dolan, 2000; Moss et al., 2005; Wagner, Maril, Bjork, & Schacter, 2001), that left dlPFC may not be necessary for the semantic task and the dissociation reported here may reflect a real difference in the neural organization of selection in prefrontal cortex.
Ventrolateral PFC was not affected by increases in verbal response selection difficulty. This may indicate a specific role in semantic selection for this region, rather than a more general top-down biasing signal (J. Jonides & Nee, 2006; Kan & Thompson-Schill, 2004) or response-related selection process (Zhang et al., 2004). Several researchers have attempted to distinguish processes within the vlPFC region. Some studies implicate anterior vlPFC in semantic retrieval (Badre et al., 2005) or semantic elaboration (Dobbins & Wagner, 2005) and mid vlPFC for semantic selection or a more general purpose selection process (Dobbins & Wagner, 2005; Thompson-Schill et al., 1997; Thompson-Schill et al., 1998). The vlPFC ROIs in the current study were located in the middle of the vlPFC across most participants, thus these data are broadly consistent with the idea that mid vlPFC mediates semantic selection. The present data provide no evidence for a role in general purpose selection process. However, it remains possible, that vlPFC may mediate a general purpose selection mechanism that was not affected by the range of response selection difficulty manipulated here.
It is important to note that the results implicating dlPFC, vlPFC and iPC in response and semantic selection mechanisms do not preclude the possibility that other regions are also involved in these processes. Many studies have reported activity in anterior cingulate and other medial PFC regions for response selection (e.g., Bunge et al., 2002; Hazeltine et al., 2003; Hester et al., 2007; Merriam et al., 2001; Rowe & Passingham, 2001; Rowe, Stephan, Friston, Frackowiak, & Passingham, 2005; Schumacher & D'Esposito, 2002) and semantic selection (e.g., Barch et al., 1999; Thompson-Schill et al., 1997). Other studies, however, failed to find significant activity in these regions (e.g., Jiang & Kanwisher, 2003; Schumacher et al., 2003; Thompson-Schill, D'Esposito, & Kan, 1999). In our participants, medial PFC regions were inconsistently active, with only a subset of participants showing supra-threshold task-related activity. For this reason, we did not investigate the effect of increasing semantic and response selection difficulty in this area. It is possible that variability in levels of activity in this region, or the use of vocal responses, precluded us from identifying these and other brain regions involved in the network for semantic and/or response selection. Nevertheless, our results do confirm a dissociation between these processes in lateral PFC.
Two final and related aims of the current study were to investigate the generality and laterality of the non-spatial response selection network identified by Schumacher and colleagues (2003). Schumacher and colleagues parametrically manipulated non-spatial response selection difficulty using digit stimuli and manual responses. They reported monotonic increases in left dlPFC and iPC activity with increases in non-spatial manual response-selection difficulty. Using verbal stimuli and vocal responses, the current data show a similar monotonic increase in these regions. This suggests that these regions mediate response selection across a variety of non-spatial tasks and response modalities.
Finally, there is a controversy in the literature about the laterality of the neural mechanisms for response selection in dlPFC for different stimulus types. Some studies report laterality differences (Schumacher et al., 2003; Schumacher et al., 2005), whereas others do not (Jiang & Kanwisher; Schumacher & D'Esposito, 2002). Our current findings provide additional evidence for hemispheric differences in response selection. We found a significant effect of verbal response selection difficulty in left dlPFC (see Figure 2). We found no corresponding increase in a homologous region in right dlPFC. In fact, there was a trend for activity to change in the opposite direction. No effect was found in right dlPFC even though the ROI tested here was within 5 mm of the site of peak activity in this region for spatial response selection from Schumacher and colleagues (2003). The significant interaction between hemisphere and response selection difficulty in dlPFC suggests hemispheric specialization for verbal response selection consistent with the hemispheric specialization for spatial and non-spatial response selection previously reported (e.g., Schumacher et al., 2003; Schumacher et al., 2005).
Taken together, the current findings provide evidence for how verbal selection is implemented in the brain and give new insights into the specificity of verbal selection brain networks. Brain mechanisms for verbal response and semantic selection rely on distinct lateral prefrontal regions but overlapping parietal regions, showing that some parts of the selection network are distinct and depend on selection type; whereas other parts of the network are common to across selection tasks. More specifically, whereas semantic selection relies mainly on vlPFC and to some extent dlPFC, response selection relies only on dlPFC. And both selection tasks involve iPC. These findings appear inconsistent with the general selection hypothesis that different kinds of selection are implemented by the same fronto-posterior brain network. Additionally, the results support the hypothesis (Schumacher et al., 2003) that the neural mechanisms for response selection are at least partially representation specific and this specialization determines the hemisphere recruited to mediate the selection of an appropriate response.
Acknowledgements
This work was supported by grants from the National Institute of Health (MH63901 and NS40813) and the Veterans Administration. Correspondence regarding this research may be addressed to: Eric H. Schumacher (eschu@gatech.edu) or Irene E. Nagel (nagel@mpibberlin.mpg.de).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ROI location in iPC was more variable than other regions. Two participants’ ROIs were more medial to Brodmann Area 40.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: Empirical investigations of problems and potential solutions. Neuroimage. 1999;10(6):642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magnetic Resonance in Medicine. 1998;40(1):55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. Journal of Neurophysiology. 1995;74(5):2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17(3):1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Neurolinguistics. 2003;16(4–5):315–339. [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gokcay D, Mohr CM, Auerbach EJ, et al. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. Journal of Cognitive Neuroscience. 2001;13(2):272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK. Event-related functional MRI: Implications for cognitive psychology. Psychological Bulletin. 1999;125(1):155–164. doi: 10.1037/0033-2909.125.1.155. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15(11):1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. "Sculpting the response space" - An account of left prefrontal activation at encoding. Neuroimage. 2000;12(4):404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2(2):157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133(1):66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JD. Material-dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia. 2003;41(9):1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Teague D, Ivry RB. Simultaneous dual-task performance reveals parallel response selection after practice. J Exp Psychol Hum Percept Perform. 2002;28(3):527–545. [PubMed] [Google Scholar]
- Hester R, D'Esposito M, Cole MW, Garavan H. Neural mechanisms for response selection: comparing selection of responses and items from working memory. Neuroimage. 2007;34(1):446–454. doi: 10.1016/j.neuroimage.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. J Cogn Neurosci. 2003;15(8):1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, et al. Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kemeny S, Ye FQ, Birn R, Braun AR. Comparison of continuous overt speech fMRI using BOLD and arterial spin labeling. Human Brain Mapping. 2005;24(3):173–183. doi: 10.1002/hbm.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: cognitive basis for stimulus-response compatibility--a model and taxonomy. Psychol Rev. 1990;97(2):253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA. Stimulus-response incompatibility activates cortex proximate to three eye fields. Neuroimage. 2001;13(5):794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 1. Basic mechanisms. Psychol Rev. 1997;104(1):3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, et al. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex. 2005;15(11):1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116(2):220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Petrides M. The role of mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14(1 Pt 1):77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15(1):85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein JM. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cognitive Science. 2003;27:525–559. [Google Scholar]
- Schumacher EH, Cole MW, D'Esposito M. Selection and maintenance of stimulus-response rules during preparation and performance of a spatial choice-reaction task. Brain Res. 2007;1136(1):77–87. doi: 10.1016/j.brainres.2006.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, D'Esposito M. Neural implementation of response selection in humans as revealed by localized effects of stimulus-response compatibility on brain activation. Hum Brain Mapp. 2002;17(3):193–201. doi: 10.1002/hbm.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D'Esposito M. Neural evidence for representation-specific response selection. J Cogn Neurosci. 2003;15(8):1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Hendricks MJ, D'Esposito M. Sustained involvement of a frontal-parietal network for spatial response selection with practice of a spatial choice-reaction task. Neuropsychologia. 2005;43(10):1444–1455. doi: 10.1016/j.neuropsychologia.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber EJ, Glass JM, Zurbriggen EL, Gmeindl L, Kieras DE, et al. Concurrent response-selection processing in dual-task performance: Evidence for adaptive executive control of task scheduling. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:791–814. [Google Scholar]
- Schumacher EH, Seymour TL, Glass JM, Fencsik DE, Lauber EJ, Kieras DE, et al. Virtually perfect time sharing in dual-task performance: uncorking the central cognitive bottleneck. Psychol Sci. 2001;12(2):101–108. doi: 10.1111/1467-9280.00318. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Feigenson K, Thompson-Schill SL. Prefrontal cortical response to conflict during semantic and phonological tasks. Journal of Cognitive Neuroscience. 2007;19(5):761–775. doi: 10.1162/jocn.2007.19.5.761. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Schumacher EH, Schubert T, D'Esposito M. The neural effect of stimulus-response modality compatibility on dual-task performance: an fMRI study. Psychological Research-Psychologische Forschung. 2006;70(6):514–525. doi: 10.1007/s00426-005-0013-7. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15(2):219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 23 Mar;1999:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage. 2006;33(3):947–957. doi: 10.1016/j.neuroimage.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. Neuroimage. 2001;14(6):1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Haughton VM, Cox RW, Hyde J, Birn RM, Wong EC, et al. Effect of motion outside the field of view on functional MR. American Journal of Neuroradiology. 1996;17(6):1005–1009. [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Feng C-M, Fox PT, Gao J-H, Tan LH. Is left inferior frontal gyrus a general mechanism for selection? Neuroimage. 2004;23:596–603. doi: 10.1016/j.neuroimage.2004.06.006. [DOI] [PubMed] [Google Scholar]