Abstract
The efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis was evaluated by applying 25 mg of each drug/kg subcutaneously every 8 h in BALB/c mice infected with N. brasiliensis. A statistically significant difference was observed only with moxifloxacin. A moxifloxacin-trimethoprim-sulfamethoxazole combination was as active as when each compound was used alone.
Mycetoma in Mexico is mainly produced by actinomycetes, with Nocardia brasiliensis being the most predominantly isolated agent in 86.6% of cases and Actinomadura madurae being the most predominantly isolated agent in 9.6% of cases. Therapy of this infectious disease is not easy. Drugs must be taken for several months, and in some cases resistance may appear (10). We present here the results of an experimental model of infection of N. brasiliensis in the BALB/c mouse using ciprofloxacin (CIP) and moxifloxacin (MXF) alone or in combination with trimethoprim-sulfamethoxazole (SXT).
(The work presented here was performed in part to fulfill the requirements for the Doctor of Medicine degree [Facultad de Medicina, Universidad Autónoma de Nuevo León] of B.E.C.-M.)
For in vitro susceptibility assays with CIP, we used a previously described broth microdilution method (9) that is based on Clinical and Laboratory Standards Institute (CLSI; formerly the NCCLS) document A-4 to evaluate 30 isolates of N. brasiliensis.
In order to determine how many bacteria are naturally resistant to the quinolones, we calculated the mutation rate of N. brasiliensis HUJEG-1 with the p0 method (5, 7) using Mueller-Hinton medium containing 1×, 4×, and 8× the MIC for each antimicrobial adjusted to contain 109 CFU per plate.
To quantitate the quinolone plasma levels in mice, we injected 8- to 12-week-old female BALB/c mice subcutaneously (s.c.) with CIP at 12.5 and 25 mg/kg and with MXF at 12.5 and 25 mg/kg as previously described (3). SXT was administered by gavage at 50 mg/kg, and blood samples were taken at 0, 20 40 60, 120, 240, 360, 480, and 600 min. SXT was also given to mice after the compound was suspended in the drinking water, and blood samples were obtained at 0, 3, 6, 9, and 12 h. The concentrations of MXF, CIP, and SXT were analyzed by using a high-pressure liquid chromatography method developed in our laboratory.
Experimental mycetomas were produced in 8- to 12-week-old female BALB/c mice using N. brasiliensis HUJEG-1 (3, 4). One week later, therapy was started. Groups of 15 animals were tested. One group of animals was injected with saline solution as a negative control. The rest were treated with MXF at 25 mg/kg s.c. three times a day; with CIP at 25 mg/kg s.c. three times a day; with MXF at 25 mg/kg s.c. three times a day plus SXT at 50 mg/kg in the drinking water twice a day; or with SXT alone at 50 mg/kg in the drinking water twice a day. The degree of infection was determined after 9 weeks (three cycles of 3 weeks of treatment and 1 week of rest) as previously described (3). Two independent evaluators scored the development of lesions, and potential differences among the groups against a control inoculated with saline solution were established by using an analysis of variance test.
The MIC range for CIP was 4 to 64 μg/ml, and the MIC50 and MIC90 were 4 and 64 μg/ml, respectively. The MICs of MXF, CIP, and SXT for N. brasiliensis HUJEG-1 were 0.25, 4, and 2.3/0.12 μg/ml, respectively.
The frequency of the in vitro appearance of antimicrobial-resistant mutants is presented in Table 1. The mutation rate among quinolones was about the same (10−9) for all quinolones at high concentrations of the antimicrobials. The addition of SXT did not modify this value significantly. Some colonies growing in the plates with a high concentration of quinolones (8×) were subcultured, and the MICs were retested. In the case of CIP, a 16-fold increase in the MIC was observed (from 4 to 64 μg/ml); the MIC of MXF increased 64-fold (from 0.25 to 16 μg/ml).
TABLE 1.
Mutation rate of N. brasiliensis HUJEG-1 in response to several quinolones and SXT
| Quinolone(s)a | Concn in agar
|
||
|---|---|---|---|
| 1× the MIC | 4× the MIC | 16× the MIC | |
| CIP | 5.3 × 10−7 | 6 × 10−9 | 1 × 10−9 |
| SPF | >1 × 10−9 | >1 × 10−9 | 8 × 10−8 |
| GAT | 7.3 × 10−7 | <1 × 10−9 | <1 × 10−9 |
| MXF | 4.8 × 10−8 | <1 × 10−9 | <1 × 10−9 |
| SXT | >1 × 10−9 | 6.9 × 10−8 | 1 × 10−9 |
| MXF + SXT | 3.4 × 10−8 | <1 × 10−9 | <1 × 10−9 |
| GAT + SXT | 2 × 10−9 | <1 × 10−9 | <1 × 10−9 |
SPF, sparfloxacin; GAT, gatifloxacin.
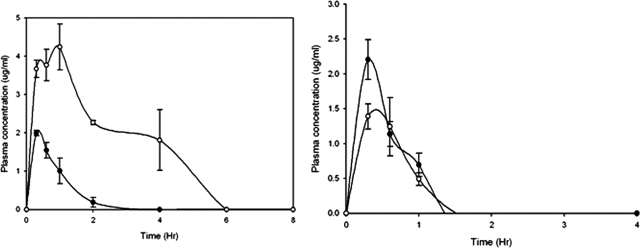
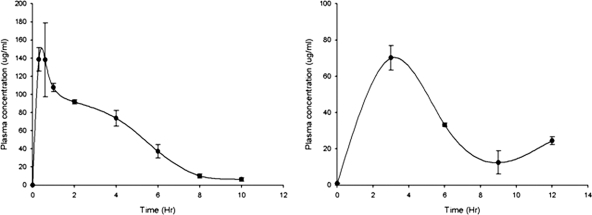
MXF plasma levels were determined at 25 and 12.5 mg/kg. At 25 mg/kg, the levels were maintained over the MXF MIC of N. brasiliensis HUJEG-1 (0.25 μg/ml) for about 5 h (Fig. 1), with a Cmax of 4.3 μg/ml. At a dose of 12.5 mg/kg the concentrations were very poor. CIP administered s.c. did not reach plasma levels greater than the MIC for N. brasiliensis HUJEG-1 (4 μg/ml), not even at the highest dose tested (25 mg/kg) (Fig. 1, right). The Cmax at 25 mg/kg was about 2.25 μg/ml. In contrast, the plasma levels of SXT administered by gavage at 50 mg/kg were sustained over the SXT MIC for N. brasiliensis HUJEG-1 (2.3/0.12 μg/ml) for about 8 h (Fig. 2). The animals taking the drug in drinking water could sustain a plasma concentration greater than the MIC for about 8 h, even though the values were lower than those observed with the gavage method.
FIG. 1.
Plasma levels produced in BALB/c mice after s.c. injection of MXF (left) at 50 and 12.5 mg/kg and of CIP (right) at the same doses. Three mice were bled and sacrificed at each point. Bars represent the standard deviation.
FIG. 2.
Sulfamethoxazole plasma levels produced in BALB/c mice after the application of SXT by gavage (left) and in drinking water (right) in both cases at 50 mg/kg. Three mice were bled and sacrificed at each point. Bars represent the standard deviation.
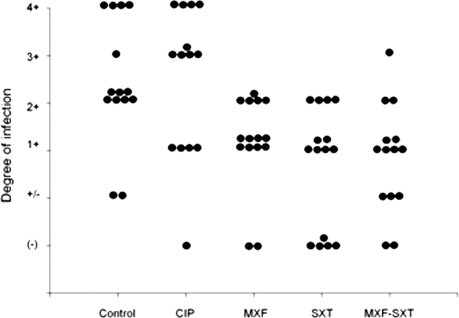
When CIP or MXF was applied s.c. (Fig. 3), only MXF had a statistically significant effect compared to the control (P = 0.017). No statistical difference was found in the CIP-treated group (P = 1.0). As shown in Fig. 3, SXT also exerted a significant effect on the development of experimental mycetoma lesions in the mouse model (P = 0.006). When SXT with MXF were combined (25 mg/kg s.c., three times a day), the effect was similar to that observed when SXT was applied alone (P = 0.007).
FIG. 3.
Effect of CIP, MXF, SXT, and the combination MXF-SXT in the development of mycetoma lesions in BALB/c mice infected with N. brasiliensis HUJEG-1. According to the analysis of variance test, significant differences were found for treatment with MXF, SXT, and MXF-SXT, with P values of 0.017, 0.006, and 0.007, respectively.
Quinolones have been widely used to treat infections by aerobic actinomycetes. CIP has been observed to be active against other species of Nocardia and mycobacteria other than tuberculosis (1, 2, 8, 12). In contrast, we observed here higher CIP MICs for N. brasiliensis than we observed with MXF and gatifloxacin. This poor in vitro susceptibility level, together with low levels of the drugs reached in mouse plasma, had no effect on the development of experimental mycetoma by N. brasiliensis.
Gatifloxacin has been shown to be active in vivo against N. brasiliensis (3). However, since there have been reports of dislycemia with this fluoroquinolone (11), it will be important to properly select young patients without diabetes before using it is used to treat actinomycetoma cases.
MXF has excellent plasma levels in humans, with a maximum plasma concentration of 6.13 at a dose of 400 mg daily and plasma levels greater than 1 μg/ml for about 8 h (6), and it has low toxicity in long-term applications. According to the results obtained here we feel MXF may be useful in the treatment of actinomycetoma using 400 mg every 12 h to keep the plasma levels greater than the MIC and to avoid the selection of naturally resistant bacteria.
Acknowledgments
This research was supported by CONACYT grant 52219.
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Alangaden, G. J., and S. A. Lerner. 1997. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clin. Infect. Dis. 25:1213-1221. [DOI] [PubMed] [Google Scholar]
- 2.Bath, P. M., K. W. Pettingale, and J. Wade. 1989. Treatment of multiple subcutaneous Nocardia asteroides abscesses with ciprofloxacin and doxycycline. Postgrad. Med. J. 65:190-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daw-Garza, A., O. Welsh, S. Said-Fernández, H. G. Lozano-Garza, N. Waksman de Torres, N. C. Rocha, J. Ocampo-Candiani, and L. Vera-Cabrera. 2008. In vivo therapeutic effect of gatifloxacin on BALB/c mice infected with Nocardia brasiliensis. Antimicrob. Agents Chemother. 52:1549-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinoza-González, N. A., O. Welsh, N. W. de Torres, N. Cavazos-Rocha, J. Ocampo-Candiani, S. Said-Fernandez, G. Lozano-Garza, S. H. Choi, and L. Vera-Cabrera. 2008. Efficacy of DA-7218, a new oxazolidinone prodrug, in the treatment of experimental actinomycetoma produced by Nocardia brasiliensis. Molecules 13:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster, P. L. 2006. Methods for determining spontaneous mutation rates. Methods Enzymol. 409:195-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peloquin, C. A., D. J. Hadad, L. P. Molino, M. Palaci, W. H. Boom, R. Dietze, and J. L. Johnson. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosche, W. A., and P. L. Foster. 2000. Determining mutation rates in bacterial populations. Methods 20:4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripodi, M. F., L. E. Adinolfi, A. Andreana, G. Sarnataro, E. Durante Mangoni, M. Gambardella, R. Casillo, C. Farina, and R. Utili. 2001. Treatment of pulmonary nocardiosis in heart-transplant patients: importance of susceptibility studies. Clin. Transplant. 15:415-420. [DOI] [PubMed] [Google Scholar]
- 9.Vera-Cabrera, L., E. Gonzalez, S. H. Choi, and O. Welsh. 2004. In vitro activities of new antimicrobials against Nocardia brasiliensis. Antimicrob. Agents Chemother. 48:602-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh, O., L. Vera-Cabrera, and M. C. Salinas-Carmona. 2007. Mycetoma. Clin. Dermatol. 25:195-202. [DOI] [PubMed] [Google Scholar]
- 11.Yadav, V., and K. Deopujari. 2006. Gatifloxacin and dysglycemia in older adults. N. Engl. J. Med. 354:2725-2726. [PubMed] [Google Scholar]
- 12.Yew, W. W., P. C. Wong, S. Y. Kwan, C. Y. Chan, and M. S. Li. 1991. Two cases of Nocardia asteroides sternotomy infection treated with ofloxacin and a review of other active antimicrobial agents. J. Infect. 23:297-302. [DOI] [PubMed] [Google Scholar]