Abstract
The pharmacokinetics and safety of BILR 355 following oral repeated dosing coadministered with low doses of ritonavir (RTV) were investigated in 12 cohorts of healthy male volunteers with a ratio of 6 to 2 for BILR 355 versus the placebo. BILR 355 was given once a day (QD) coadministered with 100 mg RTV (BILR 355/r) at 5 to 50 mg in a polyethylene glycol solution or at 50 to 250 mg as tablets. BILR 355 tablets were also dosed at 150 mg twice a day (BID) coadministered with 100 mg RTV QD or BID. Following oral dosing, BILR 355 was rapidly absorbed, with the mean time to maximum concentration of drug in serum reached within 1.3 to 5 h and a mean half-life of 16 to 20 h. BILR 355 exhibited an approximately linear pharmacokinetics for doses of 5 to 50 mg when given as a solution; in contrast, when given as tablets, BILR 355 displayed a dose-proportional pharmacokinetics, with a dose range of 50 to 100 mg; from 100 to 150 mg, a slightly downward nonlinear pharmacokinetics occurred. The exposure to BILR 355 was maximized at 150 mg and higher due to a saturated dissolution/absorption process. After oral dosing of BILR 355/r, 150/100 mg BID, the values for the maximum concentration of drug in plasma at steady state, the area under the concentration-time curve from 0 to the dose interval at steady state, and the minimum concentration of drug in serum at steady state were 1,500 ng/ml, 12,500 h·ng/ml, and 570 ng/ml, respectively, providing sufficient suppressive concentration toward human immunodeficiency virus type 1. Based on pharmacokinetic modeling along with the in vitro virologic data, several BILR 355 doses were selected for phase II trials using Monte Carlo simulations. Throughout the study, BILR 355 was safe and well tolerated.
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) include a diverse group of compounds that bind to a hydrophobic pocket in the p66 subunit of human immunodeficiency virus type 1 reverse transcriptase (HIV-1 RT). Unlike nucleoside reverse transcriptase inhibitors (NRTIs), which exert antiretroviral activity on the active site of HIV-1 RT following initial activation through intracellular phosphorylation, NNRTIs exert their antiviral activity by disrupting the conformation of the active site of HIV-1 RT through noncompetitive binding to a hydrophobic pocket distant from the active site (7). Because of their distinct mechanism of antiretroviral activity and relatively better tolerability profiles, as well as convenient dosing regimens, NNRTI-based regimens are recommended by expert panels as preferred regimens for treatment-naïve and treatment-experienced HIV-infected patients (6). Despite better tolerability and convenient dosing, the clinical utility of the current licensed NNRTIs is somewhat limited by their low genetic barrier (5, 9, 19). Compared to that in other antiretroviral agents, the selection of resistance mutations occurs relatively rapidly in NNRTIs, and mutant HIV viruses are often cross-resistant to the whole class of NNRTIs. As a result, patients failing on a current NNRTI-containing regimen are typically infected with viruses that are cross-resistant to all members of the class, leaving them with no further NNRTI options (1, 2). Thus, there is a clear need to develop a new generation of NNRTIs with potent and durable antiviral activity against both wild-type (WT) and clinically relevant NNRTI-resistant strains in order to complement and further improve existing combination therapies.
To meet this increasing need for better and newer NNRTIs, many attempts have been made by the pharmaceutical industry to develop such compounds. As a result, several promising compounds have emerged, and BILR 355 is one of the compounds that is currently under clinical development (5, 8, 21). BILR 355 displays highly specific activity toward HIV-1 RT. The 50% effective concentration (EC50) of BILR 355 against WT HIV-1 is 0.26 ng/ml and the EC50s against NNRTI-resistant viruses range from 1.5 to 13 ng/ml for the clinical common single and double NNRTI mutations (i.e., K103N, Y181C, and K103N/Y181C) (5). With EC50 values being generally lower than 10 nM (4.45 ng/ml), BILR 355 is capable of effectively suppressing viral growth against the clinical isolates with genotypes consistent with NNRTI resistance, NRTI resistance, or protease inhibitor (PI) resistances. General/safety pharmacology studies show that BILR 355 is well tolerated, with modest effect on central nervous system, cardiovascular, and renal function following high doses (100 mg/kg). The effects of BILR 355 on hERG have not been evaluated yet. In vitro metabolism studies suggest that CYP 3A4 is likely to be the major enzyme responsible for the metabolism of BILR 355 (5). Following a single oral dose of BILR 355 drink solution to healthy volunteers, the mean time to maximum concentration of drug in serum (Tmax) was 0.5 to 1.5 h and the mean terminal half-life (t1/2) was 2 to 4 h. BILR 355 exposures appear to be more than dose proportional to the increasing doses (12). After a single oral dosing of BILR 355 coadministered with 100 mg ritonavir (RTV) (BILR 355/r), the BILR 355 Tmax was delayed to 1.5 to 3 h, while the t1/2 was prolonged to 10 to 15 h. The values of the maximum concentration of drug in plasma (Cmax) and the area under the concentration-time curve from 0 h to infinity (AUC0-∞) increased 15- to 30-fold and 2- to 5-fold, respectively. The exposures to BILR 355 appear to be approximately dose proportional.
Given the favorable pharmacokinetic (PK) profiles observed following single dosing of BILR 355/r, it was decided to further characterize the PK profiles of BILR 355 after repeated dosing. The objectives of this study therefore were to evaluate BILR 355 pharmacokinetics and document the safety and tolerability of BILR 355 after multiple dosing of BILR 355/r. The pharmacokinetics data obtained from this study were to be used in conjunction with in vitro virologic data in modeling and simulation to rationally select phase II doses.
MATERIALS AND METHODS
Subjects.
The study was conducted at the Buffalo Clinical Research Center in Buffalo, NY, following approval by the local Ethics Committee. After giving informed consent, healthy male volunteers aged 18 to 60 years with a body mass index between 18.5 and 29.9 kg/m2 entered into the study. Subjects were in generally good health, as judged by medical history, physical exams, and the clinical laboratory data. Clinically abnormal results, evidence of existing diseases or disorders, or any observations or conditions (e.g., smoking, consumption of alcohols, and drug abuses) that might interfere with the pharmacokinetics of the study drug were reasons for exclusion. Subjects could be withdrawn from the study at any time due to an adverse event, an inclusion/exclusion criteria violation, a failure to show for the study, the withdrawal of consent, the intake of any concomitant drugs interfering with the study medication, or the onset of an illness. The volunteers dropped from the trial were not replaced unless the number of volunteers per dose group was less than six.
Study design.
This was a single-center, investigator-blind, randomized, placebo-controlled, multiple-dose escalation study. Originally, a total of up to 80 healthy volunteers were planned to participate in the trial, with 10 cohorts of eight subjects (six active drug [BILR 355/r] and two placebo [placebo + RTV]). An additional two dose groups were added later in the trial to explore twice a day (BID) dosing of BILR 355 and RTV. This study was divided into two segments. In segment one (including cohorts 1 to 4 [PK treatment groups S1 to S4]), a drink solution of BILR 355 was used for dosing (powder in a bottle [PIB], dissolved in PEG 400), and in segment two (including cohorts 5 to 12 [PK treatment groups T5 to T12]), a tablet form of BILR 355 was administered. In segment one, 5-, 12.5-, 25-, and 50-mg BILR 355 solutions were coadministered with 100 mg RTV once daily (QD). For cohorts 5 to 10 of segment two, a 50-, 75-, 100-, 150-, 200-, or 250-mg BILR 355 tablet was coadministered with 100 mg RTV QD. For cohorts 11 and 12 of segment two, 150 mg BILR 355 was dosed BID for both cohorts. However, for cohort 11, 100 mg RTV was dosed QD, while for cohort 12, 100 mg RTV was given BID. For cohorts 1 to 5, the healthy volunteers were dosed for 11 days. During segment two, an interim analysis was performed after the 50-mg dose to determine the time for the plasma BILR 355 concentration to reach steady state. The duration of dosing at doses higher than 50 mg was to be modified based on this analysis. As a result of this analysis, for cohorts 6 and higher, the subjects were dosed 7 days to speed up the dosing escalation process. For all cohorts, additional RTV doses were given 10 h before the first morning dose of BILR 355 (−10 h) and at the same dosing intervals after stopping dosing of BILR 355, up to 24 h or 12 h (for cohort 12 only) before the last blood sample was withdrawn. The additional doses of RTV were used to maintain the same plasma RTV levels as those in chronic treatment to allow better estimating of the t1/2 of BILR 355.
Blood and urine sampling. (i) Blood sampling.
Intensive PK samples were taken at day 1 and at steady state (day 7 or day 11). For day 1 sampling, blood samples were taken at the predose (0) hour and at 0.25, 0.5, 0.45, 1, 1.5, 2, 2.5, 3, 4, 6, 9, 10, 12, 16, and 24 h after drug application. For steady state, additional blood samples were taken at 48 and 72 h after the last drug administration. In addition, trough blood samples were taken each morning between day 1 and steady state. At each time point, about 5-ml blood samples were drawn into collection tubes containing heparin anticoagulant and bearing sample identification. The samples were centrifuged at 4°C for plasma preparation. Centrifugation was carried out within 60 min after blood sampling at 2,000 × g (equivalent to 3,000 rpm) for 10 min. Two aliquots of plasma samples (about 1.2 ml in polypropylene tubes) were available for drug assays. The plasma was stored in individually labeled polypropylene tubes at −20°C.
(ii) Urine sampling.
Urine was collected quantitatively (by a determination of volume). Two aliquots of urine (each 20 ml) were then stored at −20°C until analysis. Urine was collected for 0 to 24 h after drug administration with the following collection intervals: prior to drug administration (blank sample), 0 to 6 h, and 6 to 24 h. The bladder was voided before the beginning of each collection interval.
Bioanalytical method.
A validated high-pressure liquid chromatography-tandem mass spectrometry method was used in the assay of plasma and urine samples. An aliquot of human plasma (heparin) or urine containing BILR 355 and an internal standard was extracted using a protein precipitation/online switch valve procedure. The extracted samples were analyzed with a high-pressure liquid chromatograph equipped with a PE Sciex API 3000 mass spectrometer. Positive ions were monitored in the selected reaction-monitoring mode. Quantization was by peak area ratio. The calibration range was 2.00 ng/ml to 1,000.00 ng/ml. The lower limit of quantification was 2 ng/ml. The between-batch precision and accuracy were 4.3% to 8.7% and 0.6% to 4.8%, respectively, for plasma; and for urine, the between-batch precision and accuracy were 2.9% to 5.2% and 0.1% to 14.2%, respectively.
Safety assessment.
The safety and tolerability of BILR 355 were evaluated on the basis of adverse events, physical examinations, laboratory tests (serum chemistry, hematology, and urinalysis), measurements of vital signs, and electrocardiograms (ECG). The severity, duration, and potential relationship of the adverse events to the study drug were assessed by the investigator. All safety assessments were performed at the screening, before the first drug dose administration, and at the follow-up visits. In addition, the safety measurements (laboratory tests, vital signs, and ECG) were also taken at several predefined periods specified in the protocol. The adverse events were monitored throughout the study.
Noncompartmental PK analyses.
Plasma BILR 355 concentration-time data were analyzed by a noncompartmental approach using WinNonlin (version 4.01, Gary, NC). Cmax, Tmax, terminal elimination constant (λz), and t1/2 values were obtained through the standard WinNonlin procedure. The AUC was calculated using the linear up/log down algorithm of WinNolin. The apparent steady-state clearance, CL/Fss, was calculated as dose/AUC0-τ,ss, where F is the systemic availability and AUC0-τ,ss is the steady-state AUC over the dose interval (τ); the apparent volume of distribution, Vz/Fss, was determined as (CL/Fss)/λz. The renal clearance (CLR) was calculated as the ratio of the unchanged drug excreted in urine (Ae) over a time interval to the plasma AUC over the same interval. The fraction excreted as unchanged drug in urine (fe) was calculated as the Ae as a percentage of the administered dose. The accumulation ratio was determined as AUC0-τ,ss/AUC0-τ,1, where AUC0-τ,1 is the day 1 AUC over τ. The accumulation index was calculated as 1/(1 − e−λz·τ).
Compartmental PK modeling and Monte Carlo simulation with NONMEM.
Data collected from cohorts 3 to 6 (25-mg and 50-mg solution plus 50-mg and 75-mg tablet, QD) of this study were used for the development of a PK model describing the pharmacokinetics of BILR 355 in healthy volunteers using the NONMEM program (NONMEM version V, level 1.1, NONMEM Project Group, UCSF/GloboMax). The following two models were developed: (i) a model based on the PK data of cohorts 3 to 6 (combined data of solution and tablet cohorts) and (ii) a model based on the PK data of cohorts 5 and 6 only (tablet cohort data only). One- and two-compartment models with first-order absorption and with or without lag time were evaluated as an initial model. Log-normal distribution of all structural parameters was assumed. A constant coefficient of variation error was used to describe the residual variability. The first-order conditional evaluation method with interaction was utilized throughout the modeling process. The developed models were evaluated (validated) by employing the predictive check technique; new sets of data were simulated by using the final models, and the simulated data were compared against the experimental data to assess the performance of the models (20). The validated final model was then used in simulating different clinical trial scenarios to aid the selection of doses for phase II trials. During the modeling process, no PK parameters were fixed to predefined values.
Statistical analysis.
The power model was used for the analysis of dose proportionality in terms of the AUC and Cmax. The model is described by the following equation: Ykm = α·Dkβ·ekm. Logarithmic transformation yields the linear regression equation as follows: ln Ykm = ln α + β·ln Dk + ln ekm, where Ykm is the response (AUC, Cmax) measured for subject m receiving dose k, ln α is the intercept, β is the slope, Dk is the kth dose effect (k = 1, 2, etc.), and ekm is the random error associated with the mth subject who received the kth dose. Dose linearity requires that β equals 1. If the 95% confidence interval of β (derived from analysis of variance) contained unity, then dose proportionality would be declared. The time to reach steady state was estimated by graphical exploration and the geometric mean ratio of the successive dose-normalized trough plasma concentration. The confidence interval and P value of the geometric mean ratio were computed to assess the variability of the estimates to help determine if steady state was achieved or if the variability was too high to make any definitive conclusion.
RESULTS
Subjects.
A total of 94 eligible male subjects (70 on active drug treatment and 24 on placebo [plus RTV]) participated in the trial. The mean age varied somewhat across cohorts, ranging from 25.2 years for the 50-mg solution and 100-mg tablet groups to 47.8 years for the 5-mg solution group. All cohorts except the 5-mg solution group had a mean age ranging from 25 to 35 years, with an overall mean of 31 years old. Seventy-three percent of the subjects were white; 24% of the subjects were black; and 3% of the subjects were Asian, with the nonwhite subjects spread across multiple cohorts. The mean heights were similar across cohorts, ranging from 171.0 to 185.4 cm, with an overall mean of 178.8 cm. Mean weights were more variable across cohorts, ranging from 77.0 to 91.5 kg, with an overall mean of 85 kg. The mean body mass index was 26.5, ranging from 24.3 to 27.9. Most subjects (78.6%) reported average alcohol consumption and had never smoked (87.1%), with similar rates seen across cohorts. Overall, all baseline demographic variables were very similar for BILR 355/r and placebo subjects.
Safety and tolerability.
The overall adverse event rate during the trial was low. No death or serious adverse events are reported in this study. A total of 23 (32.9%) subjects receiving BILR 355/r, 6 (25.0%) subjects receiving placebo, and 3 (3.2%) subjects receiving RTV alone (before BILR 355/r treatment) reported adverse events. There was no apparent difference in the frequencies of adverse events between dosing levels. Overall, 11 (15.7%) of the subjects in the study drug group and 1 (4.2%) subject in the placebo/r group had adverse events which were considered by the investigator to be possibly related to study medication (either BILR 355 or RTV). The most common related adverse events in the BILR 355/r groups were diarrhea (six subjects, 8.6%) and upper abdominal pain (two subjects, 2.9%). No other events considered possibly related to treatment occurred in more than one subject. Mild increases in creatine kinase were seen across treatment groups, and some minor effects on estrone, androstenedione, and high-density lipoproteins/low-density lipoproteins should be explored in further studies with longer duration. Also, effects on liver function tests cannot be ruled out, as one subject did experience increases. There was no effect on ECG, and a mild increase in vital signs was observed in both active and placebo groups, indicating that the effect on blood pressure may be due to study activities rather than BILR 355.
Noncompartmental pharmacokinetics.
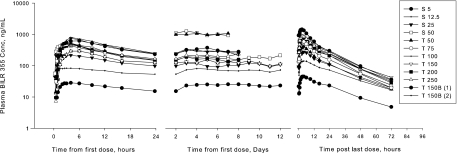
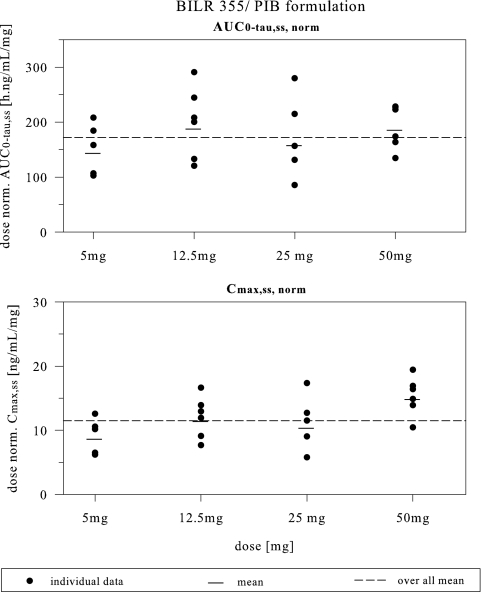
As shown in Fig. 1 and Table 1, following multiple dosing of the BILR 355 drinking solution coadministered with RTV, the mean Tmax,ss values were approximately 1 to 4 h and the mean t1/2,ss values approximately 15 to 21 h. The mean accumulation ratio ranged from 1.32 to 1.6, somewhat less than the accumulation index, indicating no excess accumulation. The mean CL/Fss was in the range of 5.42 to 7.13 liters/h. The mean fe was less than 6% during 24-h time intervals. As shown in Fig. 2, an increase in the BILR 355 dose appeared to lead to an approximate linear increase in AUC0-τ,ss and Cmax,ss over the dose range of 5 to 50 mg for the solution formulation. Statistical analysis indicated that dose proportionality can be concluded for AUC, but not for Cmax, for this dose range. As shown in Fig. 1 and confirmed by statistical analysis, the plasma BILR 355 concentration reached steady state after 5 to 6 days of dosing.
FIG. 1.
Mean plasma BILR 355 concentration time profiles after multiple oral dosing of BILR 355 coadministered with 100 mg RTV. (See Tables 1 and 2 for information regarding the PK treatment groups.)
TABLE 1.
Steady-state PK parameters of BILR 355 after multiple oral dosing of BILR 355/ra
| PKTRT | AUC0-τ,ss (ng·h/ml) | Cmax,ss (ng/ml) | Cmin,ss (ng/ml) | t1/2,ss (h) | Tmax,ss (h) | CL/Fss (liters/h) | Vz/Fss (liters) | CLR,0-24,ss (liters/h) | Fe0-24,ss (%) |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 761 ± 233 | 46 ± 14 | 23.2 ± 8.19 | 20.7 ± 1.77 | 3.6 ± 1.47 | 7.13 ± 2.27 | 209 ± 53.2 | 0.286 ± 0.165 | 3.89 ± 1.7 |
| S2 | 2,494 ± 813 | 151 ± 41 | 72.9 ± 30.4 | 21.8 ± 2.77 | 3.25 ± 1.78 | 5.53 ± 1.95 | 168 ± 40.3 | 0.33 ± 0.214 | 5.49 ± 1.73 |
| S3 | 4,339 ± 1,888 | 282 ± 108 | 108 ± 53 | 19.3 ± 0.888 | 2.9 ± 1.08 | 6.79 ± 3.15 | 188 ± 88.5 | 0.337 ± 0.15 | 5.15 ± 1.06 |
| S4 | 9,590 ± 1,991 | 767 ± 153 | 215 ± 90.2 | 15.6 ± 2.39 | 1.54 ± 1.05 | 5.42 ± 1.23 | 124 ± 41.4 | 0.31 ± 0.162 | 5.54 ± 1.7 |
| T5 | 5,215 ± 1,068 | 424 ± 86 | 112 ± 24.9 | 17.1 ± 0.681 | 3.4 ± 0.894 | 10 ± 2.55 | 248 ± 67.7 | 0.394 ± 0.157 | 3.92 ± 1.15 |
| T6 | 7,580 ± 2,188 | 643 ± 205 | 157 ± 34 | 16.1 ± 1.82 | 2.83 ± 1.03 | 10.7 ± 3.59 | 251 ± 96.8 | 0.281 ± 0.149 | 2.67 ± 1.42 |
| T7 | 9,919 ± 2,629 | 781 ± 131 | 234 ± 102 | 16.9 ± 1.4 | 4.25 ± 2.4 | 10.7 ± 2.75 | 259 ± 65.1 | 0.154 ± 0.0,213 | 1.54 ± 0.511 |
| T8 | 11,785 ± 1,972 | 870 ± 130 | 279 ± 54 | 17.2 ± 2.61 | 3.3 ± 0.671 | 13 ± 1.97 | 322 ± 74.1 | 0.173 ± 0.0,423 | 1.33 ± 0.207 |
| T9 | 10,595 ± 3,269 | 928 ± 284 | 210 ± 68 | 16.5 ± 1.71 | 2.59 ± 0.863 | 20.6 ± 6.82 | 479 ± 120 | 0.229 ± 0.125 | 1.08 ± 0.377 |
| T10 | 11,495 ± 3,243 | 919 ± 251 | 253 ± 58 | 16.8 ± 2.46 | 2.83 ± 0.931 | 23.3 ± 6.67 | 577 ± 223 | 0.194 ± 0.101 | 0.827 ± 0.39 |
| T11 | 12,260 ± 2,924 | 1,488 ± 337 | 594 ± 131 | 15.6 ± 1.73 | 2.33 ± 0.516 | 12.7 ± 2.61 | 286 ± 63.8 | 0.278 ± 0.0,939 | 3.03 ± 0.79 |
| T12 | 12,461 ± 2,851 | 1,500 ± 384 | 570 ± 101 | 14.7 ± 0.801 | 1.5 ± 0.837 | 12.6 ± 2.73 | 267 ± 59.1 | 0.255 ± 0.126 | 2.78 ± 1.36 |
PKTRT, PK treatment groups. All values are presented as three significant digits. n = 6 for all groups, except S1, S3, T5, and T8, which have 5 subjects. Treatment groups S1 to S4 represent solution cohorts 1 to 4. Treatment groups T5 to T12 represent tablet cohorts 5 to 12. S1, S2, S3, and S4 were given 5, 12.5, 25, and 50 mg of solution (PIB) QD, respectively. T5, T6, T7, T8, T9, and T10 were given 50-, 75-, 100-, 150-, 200-, and 250-mg tablets QD, respectively. T11 was given a 150-mg BILR 355 tablet BID + 100 mg RTV QD. T12 was given 150 mg BILR 355 BID + 100 mg RTV BID.
FIG. 2.
Dose-normalized (norm.) steady-state AUC0-τ,ss and Cmax,ss versus doses after multiple dosing of BILR 355 in the PIB formulation coadministered with 100 mg RTV.
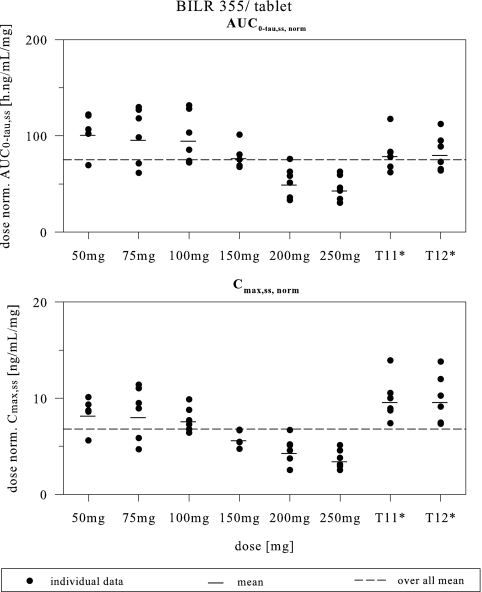
As shown in Tables 1 and 2 (for PK treatment groups T5 to T10), the mean CL/Fss was 10 to 23.3 liters/h after multiple dosing of BILR 355, a 50- to 250-mg tablet with RTV. Starting from 150 mg, the CL/Fss values were increased with increasing doses, suggesting that the systemic availability of BILR 355 declined after reaching the 150-mg dose level. The mean fe values were less than 4% for all the group. The gradual decline of fe values reflected a reduction of relative bioavailability with an increase in the dose from 50 mg to 250 mg. Figure 3 provides a visual assessment of the dose proportionality (DP) region for tablet formulation. Statistical analysis determined DP for both AUC0-τ,ss and Cmax,ss over the dose range of 50 to 100 mg; however, DP can be concluded statistically only for AUC0-τ,ss (AUC0-24 h,ss), not for Cmax,ss, for the 50-to-150-mg dosing range. For the dose range of 50 to 250 mg, DP was not concluded for either AUC0-τ or Cmax. Statistical and graphical analysis of the trough concentration (Fig. 1) showed that BILR 355 concentrations achieved steady state after approximately 5 to 6 days of dosing.
TABLE 2.
PK parameters (day 1) of BILR 355 after single oral dosing of BILR 355/ra
| PKTRT | AUC0-τ, 1 (ng·h/ml) | Cmax,1 (ng/ml) | Cmin,1 (ng/ml) | Tmax,1 (h) | CLR,0-24,1 (liters/h) | fe,0-24,1 (%) | Accumulation ratio | Accumulation index |
|---|---|---|---|---|---|---|---|---|
| S1 | 500 ± 133 | 29.5 ± 7.69 | 15.2 ± 3.42 | 3.8 ± 1.44 | 0.342 ± 0.165 | 3.21 ± 1.47 | 1.52 ± 0.202 | 1.81 ± 0.101 |
| S2 | 1,586 ± 460 | 91.8 ± 24.1 | 53.5 ± 23.8 | 2.83 ± 1.91 | 0.295 ± 0.158 | 3.3 ± 1.09 | 1.56 ± 0.203 | 1.88 ± 0.159 |
| S3 | 3,677 ± 1,649 | 272 ± 130 | 98 ± 42.3 | 0.958 ± 0.557 | 0.34 ± 0.15 | 4.21 ± 0.892 | 1.32 ± 0.169 | 1.73 ± 0.0,502 |
| S4 | 6,413 ± 2,176 | 499 ± 197 | 147 ± 49.9 | 1.92 ± 1.36 | 0.376 ± 0.184 | 4.4 ± 1.32 | 1.6 ± 0.471 | 1.53 ± 0.129 |
| T5 | 4,324 ± 1,272 | 343 ± 98.3 | 127 ± 56.6 | 4.5 ± 2.59 | 0.358 ± 0.103 | 2.92 ± 0.475 | 1.24 ± 0.268 | 1.61 ± 0.0,378 |
| T6 | 6,355 ± 2,320 | 490 ± 196 | 152 ± 39.1 | 3.58 ± 0.665 | 0.224 ± 0.106 | 1.81 ± 1.04 | 1.23 ± 0.197 | 1.55 ± 0.1 |
| T7 | 6,910 ± 1,392 | 547 ± 97.3 | 165 ± 36.8 | 4.33 ± 2.42 | 0.188 ± 0.0,347 | 1.29 ± 0.296 | 1.45 ± 0.353 | 1.6 ± 0.0,774 |
| T8 | 9,258 ± 2,894 | 663 ± 228 | 231 ± 63.5 | 4.9 ± 1.6 | 0.235 ± 0.0,876 | 1.42 ± 0.735 | 1.46 ± 0.777 | 1.61 ± 0.144 |
| T9 | 10,784 ± 3,940 | 821 ± 314 | 248 ± 98.8 | 3.33 ± 1.08 | 0.233 ± 0.104 | 1.13 ± 0.325 | 1 ± 0.144 | 1.57 ± 0.0,946 |
| T10 | 10,022 ± 2,726 | 769 ± 233 | 245 ± 44.7 | 4.17 ± 0.983 | 0.168 ± 0.0,867 | 0.635 ± 0.303 | 1.15 ± 0.109 | 1.59 ± 0.137 |
| T11 | 4,304 ± 1,171 | 1,730 ± 513 | 264 ± 68.7 | 16.0 ± 0.007 | 0.246 ± 0.104 | 1.54 ± 0.625 | 2.93 ± 0.487 | 2.42 ± 0.204 |
| T12 | 4,400 ± 1,487 | 1,412 ± 83.5 | 296 ± 71.4 | 17.3 ± 3.27 | 0.202 ± 0.0,864 | 1.15 ± 0.466 | 3.01 ± 0.975 | 2.32 ± 0.0,937 |
All values are presented as three significant digits. PKTRT, PK treatment groups. n = 6 for all groups, except S1 and T8, which have 5 subjects. Treatment groups S1 to S4 represent solution cohorts 1 to 4. Treatment groups T5 to T12 represent tablet cohorts 5 to 12. S1, S2, S3, and S4 were given 5, 12.5, 25, and 50 mg of solution (PIB) QD, respectively. T5, T6, T7, T8, T9 and T10 were given 50-, 75-, 100-, 150-, 200-, and 250-mg tablets QD, respectively. T11 was given a 150-mg BILR 355 tablet BID + 100 mg RTV QD. T12 was given a 150-mg BILR 355 BID + 100 mg RTV.
FIG. 3.
Dose-normalized (norm.) steady-state AUC0-τ,ss and Cmax,ss versus doses after multiple dosing of BILR 355 tablets coadministered with 100 mg RTV.
The mean PK parameters of BILR 355 after oral coadministration of the 150-mg tablet of BILR 355 BID with 100 mg RTV QD (cohort 11) or BID (cohort 12) are shown in Tables 1 and 2. These results demonstrated that the exposures to BILR 355 were similar, regardless of RTV being coadministered QD (cohort 11) or BID (cohort 12) with BILR 355 BID. The mean plasma BILR 355 concentration-time profiles at the troughs (Fig. 1) and the statistical analysis indicated that the plasma BILR 355 concentrations achieved steady state after approximately 5 to 6 days of dosing. It should be noted that the last mean troughs on the plots were evening troughs, which have been shown to be lower than morning troughs in both cohort 11 (927 ± 235, ng/ml) and cohort 12 (1,080 ± 283, ng/ml); the large difference (∼36 to 40%) in the plasma levels of morning and evening troughs of BILR 355 in all subjects suggested that the pharmacokinetics of BILR 355 had a diurnal variation.
For all dosing groups, the variability values for Cmax,ss and AUC0-τ,ss were in the range of 20 to 35%, and for the minimum concentration of drug in serum at steady state (Cmin,ss), the range for variability was 20 to 40%.
Modeling, simulation, and selection of doses for phase II trials.
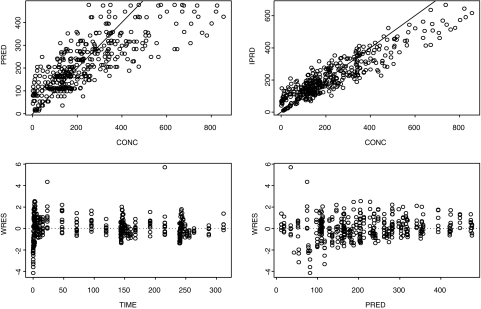
PK models based on the combined data of the solution and tablets as well as the data of tablets alone were able to adequately describe the pharmacokinetics of BILR 355. However, during the model evaluation process, the models based on tablet data were found to have better predictive performance. Given the fact that the tablet is the formulation used in the phase II trials, the PK models based on tablet data were selected for the simulations of phase II doses, and therefore, the process of development of the PK models for the tablet is presented here. The goodness-of-fit plots are depicted in Fig. 4. The PK parameters obtained from the final model are summarized in Table 3. Both one- and two-compartment models were able to successfully converge; however, based on the Akaike information criterion, the two-compartment model was selected (16). The inclusion of the absorption lag time resulted in failure to converge, and as result, it was not included in the final model. A mixture of constant variance and constant coefficient of variation residual errors was also tested but found not to be able to converge, and therefore, the proportional error model was kept as the residual error model. After taking these together, the two-compartment body model with the proportional residual error model was deemed the final model for BILR 355 tablet. The oral clearance of 9.53 liters/h estimated by compartmental modeling is in good agreement with the clearance values (CL/Fss, ∼10 liters/h) for tablet formulation of 50- to 100-mg dose groups based on noncompartmental analysis (Table 2). The standard errors of estimates were generally small compared to the parameter estimates. The predictive performance of the PK model was evaluated through the method of predictive performance check; the final model was utilized in simulating the data for all cohorts of the trial, including BID dosing, and the mean PK parameters obtained from the simulated data (n = 1,000) were presented along with the trial data in Table 4. The simulated mean PK parameters were very close to the trial data, with the majority of the difference less than 15%, suggesting that the model had a good predictive performance. The validated model was then used in simulating various scenarios in phase II trials. The summary of the simulated Cmin,ss values with distribution are presented in Table 5, as Cmin,ss values are the most relevant PK parameters in terms of projection of antiretroviral efficacy. Table 6 presents the dose selection for the phase II program, using the simulated plasma levels in combination with in vitro virological data. The simulated mean and the 10th percentile Cmin,ss values of BILR 355 are presented for each candidate dose in Table 6. The 10th percentile is the concentration that 90% of the subjects' concentrations will exceed. Protein binding-corrected EC50s for WT HIV-1 and the prototype mutant (K103N/Y181C) are presented separately in Table 6, as the susceptibility levels of these HIV-1 viruses to antiretroviral agents are different. In general, the NNRTI treatment-naïve patients are infected with wild-type HIV-1 and NNRTI treatment-experienced HIV patients are infected with mutant HIV-1. In order to maintain the suppressive concentration at all times during the dosing cycle to achieve a durable suppression of HIV-1 in both treatment-naïve and treatment-experienced HIV-infected patients, the projected Cmin,ss values have to be approximately 50-fold or more above the protein binding-corrected EC50 against HIV-1 (WT or mutant) for at least 90% of the patients. The ratios of the 10th percentile values of the Cmin,ss of BILR 355 to the EC50 values (corrected) that are presented in Table 6 provide this information. The candidate doses for treatment-naïve patients all provide sufficient margins of suppression of HIV-1 WT virus. The candidate doses for treatment-experienced patients, except 150 mg QD, all provide sufficient margins of viral suppression as well. The highest (150 mg QD and 150 BID for treatment-naïve and treatment-experienced, respectively) and the lowest (75 mg QD and 75 mg BID for treatment-naïve and treatment-experienced, respectively) doses that are projected to provide sufficient viral suppression are selected as “high” and “low” doses in the phase II trials because such selections are likely to result in distinguishable dose-response relationships (of high to low doses) to meet the goals of phase II trials.
FIG. 4.
Goodness-of-fit plots for population analysis of PK data of BILR 355 after multiple dosing of BILR 355/r. PRED, population predicted concentration (ng/ml); IPRD, individual predicted concentration (ng/ml); WRES, weighted residual; CONC, concentration (ng/ml).
TABLE 3.
PK parameters obtained from the final PK model
| Parametera | Value | SE of estimate |
|---|---|---|
| θ1, CL/F | 9.53 liters/h | 0.704 |
| θ2, V2/F | 90.9 liters | 43.3 |
| θ3, V3/F | 65.7 liters | 29.1 |
| θ4, Q | 5.47 liters/h | 1.98 |
| θ5, ka | 0.22 liters/h | 0.104 |
| ω2CL | 0.0458 | 0.0186 |
| ω2V2 | 0.144 | 0.0658 |
| ω2V3 | 0.0394 | 0.142 |
| ω2Q | 6.49E−09 | 1.41E−04 |
| ω2ka | 0.0169 | 0.0353 |
| σ12 | 0.152 | 0.0152 |
V2/F, apparent volume of distribution in the central compartment; V3/F, apparent volume of distribution in the peripheral compartment; Q, intercompartmental clearance; ka, absorption constant; ω2CL, variance of clearance; ω2V2, variance of volume of distribution in the central compartment; ω2V3, variance of volume of distribution in the peripheral compartment; ω2Q, variance of intercompartmental clearance; ω2ka, variance of absorption constant; σ12, proportional residual variance.
TABLE 4.
Predictive performance of the PK modeld
| Dose regimena | Trial data
|
Simulated data
|
% differencec
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC0-τ,ss (ng·h/ml) | Cmax,ss (ng/ml) | Cmin,ss (ng/ml) | AUC0-τ,ss (ng·h/ml) | Cmax,ss (ng/ml) | Cmin,ss (ng/ml) | AUC0-τ,ss | Cmax,ss | Cmin,ss | |
| 50 mg QD | 5,215.08 (1,068.2) | 423.6 (85.62) | 112.36 (24.95) | 5,347.26 (1,405.9) | 460.94 (115.87) | 114.64 (62.19) | 2.53 | 8.81 | 2.03 |
| 75 mg QD | 7,580.19 (2,188.45) | 642.83 (205.19) | 156.67 (33.96) | 8,020.89 (2,108.85) | 691.41 (173.8) | 171.97 (93.29) | 5.81 | 7.56 | 9.77 |
| 100 mg QD | 9,919.53 (2,628.9) | 780.5 (130.75) | 234.33 (102.32) | 10,694.52 (2,811.8) | 921.88 (231.73) | 229.29 (124.38) | 7.81 | 18.11 | −2.15 |
| 150 mg QD* | 11,785.26 (1,971.95) | 870.4 (129.62) | 278.8 (53.97) | 12,178.91 (3,202.07) | 1,049.84 (263.89) | 261.11 (141.64) | 3.34 | 20.62 | −6.35 |
| 150 mg BID* | 12,260.34 (2,924.38) | 1,488.3 (336.89) | 593.67 (131.31) | 12,041.42 (3,160.33) | 1,716.71 (442.92) | 810.51 (392.65) | −1.79 | 15.35 | 36.53b |
*, relative bioavailability was adjusted to 41% in simulation.
Evening troughs were used for comparison; if morning troughs were used, the value would be −12.52%.
Percent difference was calculated as 100·(simulated data − trial data)/trial data.
Values in parentheses indicate the standard deviations. Trial and simulated data are given to two decimal places, different from those in Table 1, which are presented as three significant digits.
TABLE 5.
Projected Cmin,ss after multiple doses of BILR 355/ra
| Dose regimenb | n | Mean | CV (%) | p90 | p75 | Median | p25 | p10 |
|---|---|---|---|---|---|---|---|---|
| 75 mg QD | 996 | 171.97 | 54.25 | 296.78 | 219.81 | 155.80 | 105.76 | 67.88 |
| 100 mg QD | 996 | 229.29 | 54.25 | 395.70 | 293.09 | 207.73 | 141.01 | 90.51 |
| 150 mg QD* | 996 | 261.12 | 54.25 | 450.63 | 333.76 | 236.56 | 160.58 | 103.07 |
| 75 mg BID | 992 | 533.79 | 48.45 | 872.81 | 682.28 | 494.91 | 354.52 | 236.21 |
| 100 mg BID | 992 | 711.72 | 48.45 | 1163.70 | 909.71 | 659.88 | 472.69 | 314.94 |
| 150 mg BID* | 992 | 810.51 | 48.45 | 1325.30 | 1035.95 | 751.47 | 538.30 | 358.65 |
CV, constant coefficient of variation. p10, p25, p75, and p90 indicate the 10th, 25th, 75th, and 90th percentiles, respectively. The values for the mean, median, and percentiles are expressed as ng/ml.
*, the relative bioavailability (to PIB) has been adjusted to 41% in simulation.
TABLE 6.
Dose selections for phase II studiesa
| HIV-1 virus | Projected dosec |
Cmin,ss (ng/ml)
|
Equation of protein-corrected EC50 (ng/ml)b | p10/EC50 ratio | |
|---|---|---|---|---|---|
| Mean | p10e | ||||
| Wild type | 75 mg QD | 171.97 | 67.88 | 0.5 × 2 = 1.0 | 68 |
| 100 mg QD | 229.29 | 90.51 | 91 | ||
| 150 mg QD | 261.12 | 103.07 | 103 | ||
| K103N/Y181C mutant | 150 mg QD | 261.12 | 103.07 | 2.0 × 2 = 4.0 | 26 |
| 75 mg BID | 392.49 | 173.68 | 43 (65)d | ||
| 100 mg BID | 523.32 | 231.57 | 58 | ||
| 150 mg BID | 595.96 | 263.71 | 66 | ||
Cmin,ss values are projected from simulation and were adjusted downward by 36% for BID dose regimens to account for the overestimation of the Cmin,ss in the model. p10, 10th percentile.
The serum shift factor is twofold. EC50 values are from in vitro data.
Each dose regimen is accompanied by 100 mg RTV QD (QD regimens) or 100 mg BID RTV (BID regimens).
The value in parentheses is the 25th percentile for 75 mg BID.
p10, 10th percentile of the projected plasma concentration.
DISCUSSION
This trial was the second clinical study with BILR 355, a second generation of NNRTI. The primary objectives of this study were to evaluate the pharmacokinetics of BILR 355 after multiple dosing of BILR 355/r and document the safety and tolerability of BILR 355. In addition, the PK data obtained in this study were to be used in modeling and simulation to assist the selection of doses for phase II trials.
BILR 355 appeared to be safe and well tolerated after 7 or 11 days of dosing of BILR 355/r, with one subject discontinued due to an adverse event (alanine aminotransferase /aspartate transaminase increase) in the 25-mg PIB group and no obvious effects on other measures of safety.
Since the lowest dose of tablet formulation started from 50 mg, the dose escalation step was started with the BILR 355 drink solution at 5 mg and then was gradually increased to higher doses of tablets. Consistent with a previous report, BILR 355 was rapidly absorbed with a mean tmax of 1.5 to 3 h; after multiple dosing of BILR 355/r, BILR 355 displayed an approximately linear dose exposure relationship in the dose range of 5 to 50 mg. The mean t1/2 of 16 to 20 h observed in the present study was somewhat longer than the half-life of 11 to 15 h following single dosing of BILR 355/r observed in the previous study (12) This may be attributed to the fact that in this study, two additional doses of RTV were given 24 and 48 h after the last BILR 355 dosing to maintain steady-state plasma RTV concentrations in the elimination phase of BILR 355, thus maintaining the boosting effect of RTV.
Following multiple dosing of BILR 355/r in tablet form (segment two of the study), exposures to BILR355 were proportional to doses up to 100 mg. From 100 to 150 mg, a slightly downward linear relationship was observed. However, from 150 mg and up, the increase in exposure relative to dose was maximized (Table 1 and Fig. 3). The limitation on the exposure to BILR 355 above 150 mg was probably attributed to a saturated dissolution/absorption process. BILR 355, a compound with high permeability but low solubility (unpublished data), may saturate the dissolution/absorption process once a large amount is taken into the gastrointestinal tract.
Compared to the 150/100 mg BILR 355/r QD, the mean daily AUC0-24 value was doubled, while the mean Cmin,ss was increased to 570 ng/ml, 66-fold higher than the protein-binding-corrected BILR 355 EC50 (Tables 1 and 6) against the prototype mutant HIV-1 (K103N/Y181C) after BILR 355 BID with low-dose RTV. It is noteworthy that the steady-state exposures to BILR 355 (AUC0-τ, Cmax, and Cmin) were comparable regardless of RTV being given as QD or BID in cohorts 11 and 12 (Table 1). RTV, a CYP 3A4 inhibitor, has been used widely as a PK enhancement agent to improve the PK profiles by inhibiting CYP p450 3A4, an enzyme responsible for the metabolism of many antiretroviral agents, such as HIV PIs (15, 18). It has been shown that in many cases low doses of RTV are as effective as the higher doses of RTV in boosting PK profiles, indicating that after reaching certain plasma levels, an extra amount of the RTV does not necessarily contribute to additional appreciable boosting effects (18). In the present study, the plasma RTV concentrations after multiple dosing QD may have already reached the levels capable of inhibiting CYP 3A4 to a maximum extent, and therefore, an additional 100-mg dose of RTV was unable to further boost plasma BILR 355 levels.
RTV PK has a diurnal variation (11). If RTV is given QD, it should be given with BILR 355 in a morning dosing to provide maximal boosting effect. While RTV provides significant boosting effect (given QD or BID) on the pharmacokinetics of BILR 355, the potential drawback of the RTV boosting NNRTIs is that if the regimen fails, patients may develop a virus resistant toward NNRTIs and PIs.
In the present study, a diurnal variation in pharmacokinetics was observed for the BILR 355 Cmin,ss in cohort 11 (594 ng/ml for the p.m. trough versus 927 ng/ml for the a.m. trough) and cohort 12 (570 ng/ml for the p.m. trough versus 1,080 ng/ml for the a.m. trough). Diurnal variation has been shown in the pharmacokinetics of RTV (11) as well as other antiretroviral agents, such as nelfinavir and delavirdine (13, 17). The cause of the variation has not been completely elucidated yet; however, it is believed that the diurnal variations in pharmacokinetics may be the result of the reduction in the hepatic blood flow during sleep, caused by the combined lowering of blood pressure and the pause rate, and the reduction of hepatic flow likely reduces the metabolism rate. Variation in the intake of carbohydrates and fat could also be an explanation (13). Since the antiviral activity of NNRTIs is related to the Cmin,ss, the diurnal variation of plasma levels of BILR 355 is likely to be clinically relevant (17), even though it has been shown by PK modeling that the time period during which evening plasma levels are below the levels of the morning trough is merely a brief one (11).
In the current study, the observed intersubject variability for the Cmax,ss and AUC0-τ,ss was in the range of 20 to 35%; for the Cmin,ss, the range was 20 to 40%. BILR 355, therefore, is considered a drug with “normal” PK variability (3).
The compartmental PK modeling reported here was performed during the double-blind dose escalation phase of the trial, which was before the availability of the demographic and the PK data of BID dosing (although the BID data were later utilized in the evaluation of the models). The results of modeling and simulation have been used in the selection of doses for phase II trials, and these selections have been submitted to the health authorities. Here we present results as they were in the submission, and therefore, they may be slightly different from the conventional modeling process. Considering the dose linearity region, only data from cohorts 3 to 6 were selected for building the model, and cohort 7 (100-mg tablet) was saved for the validation of the model. As shown in Table 4, the predictive performance of the final model was quite good, as the simulated mean PK parameter values were in good agreement with the observed data. The only exception was the Cmin,ss of BID dosing, which was 36% higher in simulated data. However, the deviation is understandable, as the PK model was built based on the QD data; it is unlikely to predict the diurnal effects which could be measured only following BID dosing. A 36% downward adjustment for the simulated Cmin,ss was thus later used in accounting for the diurnal variation of the pharmacokinetics of BILR 355. The variability in the simulated data was 25 to 27% for the Cmax and AUC and 48 to 54% for the Cmin,ss, values that were somewhat higher than the observed data. Because the phase II trials will be conducted in HIV-infected male and female patients, a larger intersubject variability than those observed in this study is expected. A 40 to 50% intersubject variability in trough levels was reported for nevirapine (14), an NNRTI structurally similar to BILR 355, suggesting that a variability of 48 to 50% for the Cmin,ss for BILR 355 in HIV-infected patients is a reasonable assumption in projecting phase II doses. Apparently, when phase II data become available, it will be necessary to refine the PK model by incorporating the demographic data and patient disease characteristics, as well as pharmacodynamic data, to simulate the phase III doses in aiding the design of the pivotal trials.
The current study shows that even though some uncertainties (such as variability in the plasma levels of HIV-infected patients) exist in the simulated data, Monte Carlo simulations can be used in exploring the different scenarios of clinical trials, particularly the ones for which limited experimental data are available, such as the BID dosing regimens in the current study. As a result, after comprehensive analysis of the outcome of the simulated trials, an optimal design can be obtained, and thus, a higher likelihood of success for the clinical trial is expected. Application of Monte Carlo simulations in aiding the design of clinical trials is a new method that has gained popularity in recent years, and apparently, more research is needed in this field (4, 10).
In conclusion, the present study demonstrates that following multiple dosing of BILR 355/r, BILR 355 is safe and well tolerated. BILR 355 is rapidly absorbed, with a mean Tmax of 1.3 to 5 h and a mean t1/2 of 16 to 20 h. For the tablet formulation, the BILR 355 exposures are approximately linear for doses from 50 to 150 mg. After oral administration of 150/100 mg BILR 355/r BID, the plasma BILR 355 levels provide sufficient suppressive concentration toward HIV-1. The BILR 355 PK data can be best described by a two-compartment model with first-order absorption and first-order elimination. Based on this model along with the in vitro virologic data, BID (75 and 150 mg) and QD (75 and 150 mg) are selected as the doses for treatment-experienced and treatment-naïve patients, respectively, for phase II trials by using Monte Carlo simulations.
Acknowledgments
This research was funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Antinori, A., M. Zaccarelli, A. Cingolani, F. Forbici, M. G. Rizzo, M. P. Trotta, G. S. Di, P. Narciso, A. Ammassari, E. Girardi, A. De Luca, and C. F. Perno. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res. Hum. Retrovir. 18:835-838. [DOI] [PubMed] [Google Scholar]
- 2.Bacheler, L., S. Jeffrey, G. Hanna, R. D'Aquila, L. Wallace, K. Logue, B. Cordova, K. Hertogs, B. Larder, R. Buckery, D. Baker, K. Gallagher, H. Scarnati, R. Tritch, and C. Rizzo. 2001. Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J. Virol. 75:4999-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blume, H. H., and K. K. Midha. 1993. Bio-International 92, conference on bioavailability, bioequivalence, and pharmacokinetic studies. J. Pharm. Sci. 82:1186-1189. [DOI] [PubMed] [Google Scholar]
- 4.Bonate, P. L. 2000. Clinical trial simulation in drug development. Pharm. Res. 17:252-256. [DOI] [PubMed] [Google Scholar]
- 5.Boone, L. R. 2006. Next-generation HIV-1 non-nucleoside reverse transcriptase inhibitors. Curr. Opin. Investig. Drugs 7:128-135. [PubMed] [Google Scholar]
- 6.Brunton, L. L., J. S. Lazo, and K. L. Parker. 2006. Goodman and Gilman's the pharmacological basis of therapeutics, 11th ed. McGraw-Hill, Inc., New York, NY.
- 7.De Clercq, E. 1996. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) for the treatment of human immunodeficiency virus type 1 (HIV-1) infections: strategies to overcome drug resistance development. Med. Res. Rev. 16:125-157. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E. 2004. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem. Biodivers. 1:44-64. [DOI] [PubMed] [Google Scholar]
- 9.Deeks, S. G. 2001. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J. Acquir. Immune Defic. Syndr. 26(Suppl. 1):S25-S33. [DOI] [PubMed] [Google Scholar]
- 10.Holford, N. H., H. C. Kimko, J. P. Monteleone, and C. C. Peck. 2000. Simulation of clinical trials. Annu. Rev. Pharmacol. Toxicol. 40:209-234. [DOI] [PubMed] [Google Scholar]
- 11.Hsu, A., G. R. Granneman, G. Witt, C. Locke, J. Denissen, A. Molla, J. Valdes, J. Smith, K. Erdman, N. Lyons, P. Niu, J. P. Decourt, J. B. Fourtillan, J. Girault, and J. M. Leonard. 1997. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 41:898-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, F., M. Koenen-Bergmann, T. MacGregor, P. Robinson, A. Ring, S. Hattox, and D. Mayers. 2006. Pharmacokinetics of BILR 355 after single ascending doses alone and in combination with 100 mg ritonavir (RTV) in healthy volunteers, abstr. 58A. Seventh Int. Workshop Clin. Pharmacol. HIV Ther. Lisbon, Portugal, 20 to 22 April 2006.
- 13.Justesen, U. S., and C. Pedersen. 2002. Diurnal variation of plasma protease inhibitor concentrations. AIDS 16:2487-2489. [DOI] [PubMed] [Google Scholar]
- 14.Kappelhoff, B. S., A. D. Huitema, F. van Leth, P. A. Robinson, T. R. MacGregor, J. M. Lange, and J. H. Beijnen. 2005. Pharmacokinetics of nevirapine: once-daily versus twice-daily dosing in the 2NN study. HIV Clin. Trials 6:254-261. [DOI] [PubMed] [Google Scholar]
- 15.King, J. R., H. Wynn, R. Brundage, and E. P. Acosta. 2004. Pharmacokinetic enhancement of protease inhibitor therapy. Clin. Pharmacokinet. 43:291-310. [DOI] [PubMed] [Google Scholar]
- 16.Ludden, T. M., S. L. Beal, and L. B. Sheiner. 1994. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J. Pharmacokinet. Biopharm. 22:431-445. [DOI] [PubMed] [Google Scholar]
- 17.Smith, P. F., R. Dicenzo, A. Forrest, M. Shelton, G. Friedland, M. Para, R. Pollard, M. Fischl, R. DiFrancesco, and G. D. Morse. 2005. Population pharmacokinetics of delavirdine and N-delavirdine in HIV-infected individuals. Clin. Pharmacokinet. 44:99-109. [DOI] [PubMed] [Google Scholar]
- 18.van Heeswijk, R. P., A. Veldkamp, J. W. Mulder, P. L. Meenhorst, J. M. Lange, J. H. Beijnen, and R. M. Hoetelmans. 2001. Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antivir. Ther. 6:201-229. [PubMed] [Google Scholar]
- 19.Wainberg, M. A. 2003. HIV resistance to nevirapine and other non-nucleoside reverse transcriptase inhibitors. J. Acquir. Immune Defic. Syndr. 34(Suppl. 1):S2-S7. [DOI] [PubMed] [Google Scholar]
- 20.Yano, Y., S. L. Beal, and L. B. Sheiner. 2001. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J. Pharmacokinet. Pharmacodyn. 28:171-192. [DOI] [PubMed] [Google Scholar]
- 21.Yoakim, C., P. R. Bonneau, R. Deziel, L. Doyon, J. Duan, I. Guse, S. Landry, E. Malenfant, J. Naud, W. W. Ogilvie, J. A. O'Meara, R. Plante, B. Simoneau, B. Thavonekham, M. Bos, and M. G. Cordingley. 2004. Novel nevirapine-like inhibitors with improved activity against NNRTI-resistant HIV: 8-heteroarylthiomethyldipyridodiazepinone derivatives. Bioorg. Med. Chem. Lett. 14:739-742. [DOI] [PubMed] [Google Scholar]