Abstract
Bacterial biofilms are resistant to conventional antimicrobial agents. Prior in vitro studies have shown that electrical current (EC) enhances the activities of aminoglycosides, quinolones, and oxytetracycline against Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus epidermidis, Escherichia coli, and Streptococcus gordonii. This phenomenon, known as the bioelectric effect, has been only partially defined. The purpose of this work was to study the in vitro bioelectric effect on the activities of 11 antimicrobial agents representing a variety of different classes against P. aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), and S. epidermidis. An eight-channel current generator/controller and eight chambers delivering a continuous flow of fresh medium with or without antimicrobial agents and/or EC to biofilm-coated coupons were used. No significant decreases in the numbers of log10 CFU/cm2 were seen after exposure to antimicrobial agents alone, with the exception of a 4.57-log-unit reduction for S. epidermidis and trimethoprim-sulfamethoxazole. We detected a statistically significant bioelectric effect when vancomycin plus 2,000 microamperes EC were used against MRSA biofilms (P = 0.04) and when daptomycin and erythromycin were used in combination with 200 or 2,000 microamperes EC against S. epidermidis biofilms (P = 0.02 and 0.0004, respectively). The results of these experiments indicate that the enhancement of the activity of antimicrobial agents against biofilm organisms by EC is not a generalizable phenomenon across microorganisms and antimicrobial agents.
Bacteria growing in biofilms cause a wide range of human infections (11). Biofilm bacteria are resistant to antimicrobics at levels 500 to 5,000 times higher than those needed to kill nonbiofilm bacteria (2, 8). The most effective way to eradicate such infections is the removal of colonized foreign bodies; but removal carries significant morbidity, cost, and, occasionally, mortality. Limited in vitro experiments have shown that electrical current (EC) enhances the activities of aminoglycosides, quinolones, and oxytetracycline against Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus epidermidis, Escherichia coli, and Streptococcus gordonii biofilms, a phenomenon referred to as the bioelectric effect (6, 9, 12, 13, 14, 18, 20, 21). The bioelectric effect was initially described for P. aeruginosa and biocides (1.5% isothialazone, 50% dimethyl ammonium chloride, and 25% glutaraldehyde) (3), P. aeruginosa and tobramycin (5 to 100 mg/liter) and ciprofloxacin (1.25 to 5 mg/liter), E. coli and tobramycin (10 to 100 mg/liter), S. epidermidis and tobramycin (2.5 to 100 mg/liter), and Candida albicans and cycloheximide (100 mg/liter) (14). Subsequently, electrically enhanced biofilm susceptibility was shown with S. gordonii and gentamicin (2 mg/liter) (20) and E. coli and gentamicin (5 mg/liter) or oxytetracycline (50 mg/liter) (6).
The aim of this study was to determine whether the in vitro enhancement of killing of biofilm-associated P. aeruginosa and S. epidermidis by EC plus aminoglycoside, quinolone, and tetracycline antimicrobial agents generalizes to antimicrobial agents representing a variety of antimicrobial classes (cephalosporin, oxazolidinone, sulfonamide, macrolide, cyclic lipopeptide, and ansamycin antimicrobial agents) and to methicillin-resistant Staphylococcus aureus (MRSA). We tested the hypothesis that the in vitro application of EC significantly increases the killing of biofilm-associated bacteria by these antimicrobial agents.
(This work was presented, in part, at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, September 2007.)
MATERIALS AND METHODS
Microorganisms.
MRSA Xen 30, S. epidermidis Xen 43, and P. aeruginosa Xen 5 (Xenogen corp., Hopkinton, MA) were studied. The strains were stored at −70°C. The MICs of the antimicrobial agents studied against the microorganisms in their planktonic state were determined by the broth microdilution method outlined by the CLSI (Table 1). The activities of moxifloxacin (Bayer Corp., West Haven, CT), cefazolin (Sigma-Aldrich Chemical Co., St. Louis, MO), minocycline (Sigma-Aldrich Chemical Co.), linezolid (Pfizer Inc., New York, NY), trimethoprim-sulfamethoxazole (Sigma-Aldrich Chemical Co.), erythromycin (Sigma-Aldrich Chemical Co.), rifampin (rifampicin; Sigma-Aldrich Chemical Co.), vancomycin (Lilly & Co., Indianapolis, IN), tobramycin (Lilly & Co), and daptomycin (Cubist Pharmaceutical Inc., Lexington, MA) against S. epidermidis and MRSA biofilms were studied. The activities of ciprofloxacin (Bayer Corp.), tobramycin (Lilly & Co), and cefepime (Bristol-Myers Squibb Corp., Princeton, NJ) against P. aeruginosa biofilms were studied.
TABLE 1.
MICs and concentrations of antimicrobial agents used in the studies
| Antimicrobial agent |
P. aeruginosa Xen 5
|
S. aureus Xen 30
|
S. epidermidis Xen 43
|
|||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | Concn used (μg/ml) | MIC (μg/ml) | Concn used (μg/ml) | MIC (μg/ml) | Concn used (μg/ml) | |
| Cefepime | 2 | 32 | ||||
| Ciprofloxacin | 0.125 | 4 | ||||
| Tobramycin | 1 | 1 | 4 | 8 | 0.25 | 8 |
| Daptomycin | 1 | 2 | 0.125 | 4 | ||
| Erythromycin | 0.5 | 2 | 0.125 | 2 | ||
| Linezolid | 2 | 32 | 1 | 32 | ||
| Minocycline | 4 | 4 | 1 | 4 | ||
| Moxifloxacin | 2 | 4 | ≤0.125 | 4 | ||
| Rifampin | 2 | 16 | ≤0.125 | 8 | ||
| Trimethoprim-sulfamethoxazole | 0.25/4.75 | 0.25/4.75 | 0.25/4.75 | 0.25/4.75 | ||
| Vancomycin | 2 | 32 | 2 | 32 | ||
Substrate solution.
Biofilms were grown on Teflon coupons in a semisynthetic medium containing 426 mg Na2HPO4, 205 mg KH2PO4, 435 mg KNO3, 32 mg MgSO4, 32 mg CaCO3, 6.4 mg nitriloacetic acid, 5 mg FeSO4, 4.5 mg ZnSO4, 0.3 mg MnSO4, 0.09 mg CuSO4, 0.07 mg Co(NO3), 0.04 mg NaB4O7, 0.05 mg (NH4)2MoO4, and 100 ml Trypticase soy broth per liter of distilled water. The solution was sterilized for 20 min at 120°C, and 640 mg of filter-sterilized glucose solution (1 mg/ml) was then added.
Biofilm growth reactor.
Biofilms were grown on Teflon disks (12.5 mm in diameter by 1 mm in thickness) in a CDC biofilm reactor (Biosurface Technologies, Bozeman, MT). The coupons were immersed vertically in 300 ml substrate solution. One milliliter of the microorganism (108 CFU/ml) was added to the reactor. The biofilm reactor was incubated at 37°C on a continuous shaker for 18 h. After 18 h of incubation, the biofilm reactor was incubated for an additional 18 h at room temperature. Then, nine coupons were aseptically removed from the reactor and used for in vitro testing.
Biofilm treatment device.
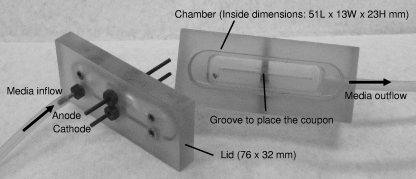
The Mayo Division of Engineering designed and fabricated two eight-channel current generators/controllers and 16 test chambers to deliver a continuous flow of fresh medium (with or without antimicrobial agent) and with or without EC to the biofilm-coated coupons. Each current controller was computer controlled to deliver a specified direct current (20 to 3,500 microamperes) and to monitor and record the voltage and current at 5-s intervals. The chambers were machined from a solid, rectangular block of polycarbonate with outside dimensions of 76 mm in length by 32 mm in width by 37 mm in height. An end mill was used to remove material measuring 51 mm in length by 13 mm in width by 23 mm in height (approximately 16 cm3). A rectangular (76- by 32-mm) piece of polycarbonate 14 mm thick formed the lid. Three holes were drilled through the lid, and brass fittings and compression gaskets were installed in each hole, forming three sealed ports. Two ports were used for the anode and cathode, and one was used for medium inflow and outflow. A single port was placed on the end of the chamber opposite the inflow port for outflow. The holes for the electrodes were positioned so that the electrodes were placed vertically in the midline of each chamber 1 cm from the end of each chamber (Fig. 1). The electrodes were 55-mm-long stainless steel or graphite cylinders of 1.5 mm in diameter. One centimeter of the electrode extended above the chamber to connect the electrode to the current generator.
FIG. 1.
Polycarbonate chamber detail (internal volume, approximately 16 cm3). L, length; W, width; H, height.
For each experiment, biofilm-covered coupons were removed from the reactor, rinsed of planktonic bacteria with 15 ml of saline, and placed in a chamber in a vertical position in a plane perpendicular to the plane formed by the electrodes. Phosphate-buffered saline plus glucose (with or without antimicrobial agent) was continuously pumped (3 ml/h) through each chamber. For each test, EC (20, 200, or 2,000 microamperes of direct current) was continuously passed from the anode to the cathode in the chamber. Each run of eight chambers was set up to expose the coupons to no antimicrobial plus 20 microamperes, no antimicrobial plus 200 microamperes, no antimicrobial plus 2,000 microamperes, antimicrobial plus 20 microamperes, antimicrobial plus 200 microamperes, antimicrobial plus 2,000 microamperes, antimicrobial plus 0 microamperes, and no antimicrobial plus 0 microamperes. A single coupon from each reactor was quantitatively cultured for determination of the density of biofilm bacteria at the time that chamber exposure was initiated. Exposure to antimicrobials and/or EC was applied for 24 h at room temperature. After exposure, the coupons were aseptically removed from the chambers, rinsed of planktonic bacteria, and placed in sterile tubes containing 1 ml of Trypticase soy broth. Adherent biofilm bacteria were removed by vortexing for 30 s and then sonication in an ultrasound bath (40 kHz, 320 mW/cm2) for 5 min. Suspensions of dislodged and disaggregated biofilms were serially diluted, and 100 μl of each dilution was spread on the surface of blood agar plates. After incubation at 37°C in 5% CO2 for 48 h, the colonies on plates containing 10 to 100 CFU were counted, and the CFU/cm2 was calculated. Each test was performed in triplicate, and the results were expressed as the mean log10 CFU/cm2 of three different experiments. The effect of the exposure was measured by using the logarithmic reduction factor (LRF) in the numbers of CFU/cm2, i.e., log [(mean CFU/cm2 of control coupons)/(mean CFU/cm2 of treated coupons)], as described elsewhere (6).
Statistical methods.
We performed a one-way analysis of variance with three levels of EC exposure of the coupons (i.e., exposure to 20, 200, or 2,000 microamperes) and no EC exposure (LRF = 0) to determine if exposure to EC alone had any effect on the biofilms. We then performed a one-way analysis of variance with four combinations of exposure (i.e., antimicrobial alone, antimicrobial plus 20 microamperes, antimicrobial plus 200 microamperes, and antimicrobial plus 2,000 microamperes) to look for a statistically significant bioelectric effect. This approach allowed us to assess whether there was any significant difference in the effects among the four levels and then to explore where such differences existed, based on pairwise comparisons. Adjustments for multiple comparisons were based on the Tukey-Kramer test. Our approach also allowed the visual comparison of the mean scores from our LFR measurements of interest and determination of the best combinations for each microorganism and treatment type (regardless of statistical significance). We subsequently compared the LRFs among the coupons exposed to an antimicrobial agent alone and an antimicrobial plus EC using the Wilcoxon rank sum test. This approach allowed us to detect which antimicrobials had enhanced activity when they were used in combination with EC (i.e., a bioelectric effect). All the tests were two sided, and P values of <0.05 were considered statistically significant. Analysis was performed with SAS software (version 9; SAS Institute, Inc., Cary, NC).
RESULTS
By using the biofilm growth reactor and the protocols described above, P. aeruginosa, MRSA, and S. epidermidis biofilms were grown on nine coupons per reactor. In preliminary experiments, there were slight differences in the mean counts between reactors, but for a given reactor there were no significant differences among the CFU/cm2 counts for each of the nine coupons (i.e., standard deviations about the mean, <5% of the mean value). Prior to each run, we verified that the density of the biofilm bacteria on one of each reactor's coupon sets was >5 log10 CFU/cm2.
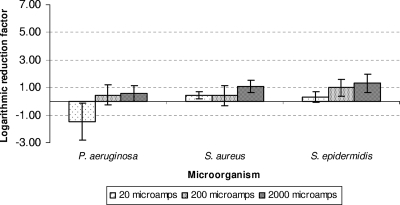
Mean LRF and standard deviation for each biofilm after exposure to the different treatments are shown in Fig. 2 to 5, and the results of the statistical analyses are shown in Tables 2 and 3. When colonized coupons were exposed to the antimicrobial agents in the absence of EC, no significant decreases in the number of CFU were seen, with the exception of an LRF of 4.57 for trimethoprim-sulfamethoxazole for S. epidermidis. LRFs ranged from −0.63 (ciprofloxacin) to 1.69 (tobramycin) for P. aeruginosa biofilms, −0.31 (vancomycin) to 1.63 (daptomycin) for MRSA biofilms, and −0.41 (moxifloxacin) to 0.7 (vancomycin) for S. epidermidis biofilms.
FIG. 2.
Mean LRFs and standard deviations for Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis after exposure to different intensities of EC (20, 200, and 2,000 microamperes). The non-EC-exposed coupons were taken as a reference (i.e., LRF = 0 on the x axis).
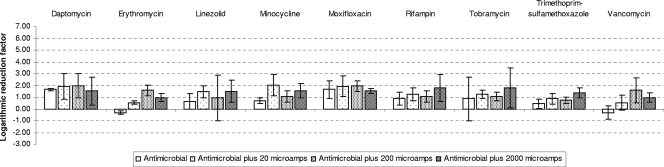
FIG. 5.
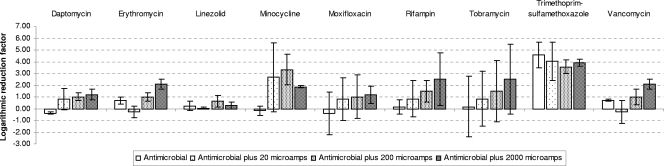
Mean LRFs and standard deviations for Staphylococcus epidermidis after exposure to four different treatments (i.e., antimicrobial agent alone or antimicrobial agent plus 20, 200, or 2,000 microamperes EC).
TABLE 2.
LRFs and statistical significances by comparison of antimicrobial agent exposure alone and antimicrobial agent plus EC combinations against Pseudomonas aeruginosa biofilms
| Antimicrobial agent | P value for difference between antimicrobial agent alone and combination treatmenta | LRF
|
|
|---|---|---|---|
| Antimicrobial alone | Best treatment | ||
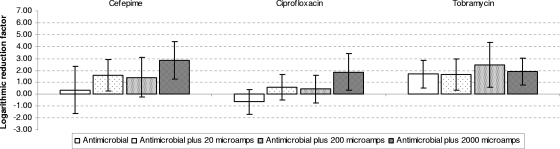
| Cefepime | 0.77 | 0.96 | 1.43b |
| Ciprofloxacin | 0.17 | −0.63 | 1.86b |
| Tobramycin | 0.87 | 1.69 | 2.48c |
There no differences between the effect of the antimicrobial agent alone and that of treatment with the combination of the antimicrobial agent plus EC (20, 200 and 2,000 microamperes).
The best treatment was the antimicrobial agent and EC at 2,000 microamperes.
The best treatment was the antimicrobial agent and EC at 200 microamperes.
TABLE 3.
LRFs and statistical significances by comparison of antimicrobial agent exposure alone and antimicrobial agent plus EC combinations against Staphylococcus and Staphylococcus epidermidis biofilms
| Antimicrobial agent |
S. aureus
|
S. epidermidis
|
||||
|---|---|---|---|---|---|---|
| Difference between treatment groups (antimicrobial agent alone, combination treatments) (P value)a | LRF
|
Difference between treatment groups (antimicrobial agent alone, combination treatments) (P value)a | LRF
|
|||
| Antimicrobial alone | Best treatment | Antimicrobial alone | Best treatment | |||
| Tobramycin | No (0.45) | 0.86 | 2 (ABX-2,000) | No (0.46) | 0.2 | 3.08 (ABX-2,000) |
| Daptomycin | No (0.9) | 1.63 | 1.94 (ABX-200) | Yes (0.02; ABX/ABX-200 and ABX/ABX-2,000) | −0.38 | 1. 2 (ABX-2,000) |
| Erythromycin | No (0.36) | −0.17 | 0.27 (ABX-2,000) | Yes (0.0004; ABX/ABX-200, ABX/ABX-2,000, ABX-20/ABX-200, and ABX-20/ABX-2,000) | 0.7 | 2.04 (ABX-200) |
| Linezolid | No (0.74) | 0.62 | 1.51 (ABX-2,000) | No (0.31) | 0.22 | 0.63 (ABX-200) |
| Minocycline | No (0.11) | 0.7 | 2.04 (ABX-20) | No (0.11) | −0.18 | 3.32 (ABX-200) |
| Moxifloxacin | No (0.4) | 1.33 | 2.1 (ABX-20) | No (0.79) | −0.41 | 0.82 ABX-2,000) |
| Rifampin | No (0.4) | 0.86 | 1.78 (ABX-2,000) | No (0.32) | 0.17 | 2.50 (ABX-2,000) |
| Trimethoprim- sulfamethoxazole | No (0.12) | 0.45 | 1.35 (ABX-2,000) | No (0.6) | 4.57 | 4.57 (ABX) |
| Vancomycin | Yes (0.05; ABX/ABX-2,000) | −0.31 | 1.59 (ABX-200) | Yes (0.01; ABX-20/ABX-2,000) | 0.7 | 2.09 (ABX-2,000) |
If significant differences existed between the group exposed to the antimicrobial agent alone and any of the combinations of antimicrobial agent plus EC, the group(s) is indicated. ABX, antimicrobial agent; 20, 200, and 2,000, 20, 200, and 2,000 microamperes EC, respectively.
When the colonized coupons were exposed to EC (20, 200, or 2,000 microamperes), we detected statistically significant differences for MRSA and S. epidermidis when the results for no EC exposure were compared with those for exposure to 20 or 2,000 microamperes alone (P < 0.0001 in both cases). There were statistically significant differences between the results for no EC exposure and those for exposure to EC at 200 or 2,000 microamperes for both microorganisms (P < 0.05). No differences were found when P. aeruginosa was not exposed to EC and when it was exposed to EC alone (P = 0.23).
No combination was statistically better than any other in the cases of cefepime, ciprofloxacin, and tobramycin plus EC against P. aeruginosa biofilms, although antimicrobials plus 200 to 2,000 microamperes were the best treatments, with LFRs ranging from 1.43 for cefepime to 2.48 for tobramycin.
We detected statistically significant differences among the treatments when vancomycin plus 2,000 microamperes EC was used against MRSA biofilms (P = 0.04). The LRFs of the different combinations ranged from 0.27 (erythromycin) to 2.04 (minocycline) for MRSA biofilms.
We detected statistically significant differences in the results when daptomycin and erythromycin were used in combination with 200 or 2,000 microamperes EC against S. epidermidis biofilms (P = 0.02 and 0.0004, respectively). We also detected differences between the resutls for combination treatments when vancomycin was used against S. epidermidis biofilms (i.e., vancomycin plus 20 microamperes of EC versus vancomycin plus 2,000 microamperes of EC) (P = 0.01); however, no differences in effects were detected when the effect of vancomycin alone was compared with the effect of any vancomycin combination treatment. The LRFs of the combination treatments ranged from 0.63 for linezolid to 3.32 for minocycline.
DISCUSSION
In today's health care environment, biofilms are present in more than 65% of all bacterial infections (15). This includes both device-related infections and chronic non-device-related infections. The inherent resistance of biofilm bacteria to antimicrobial agents is associated with the poor response observed when device-related infections are treated with antimicrobial agents alone. Costerton et al. (9) have shown that the efficacy of biocides and antimicrobial agents in the killing of bacteria can be enhanced if these agents are used within a low-intensity electric field. Much has been hypothesized to explain the mechanism of action of the bioelectric effect; however, a satisfactory explanation remains to be shown. The bioelectric effect may be related to pH modifications, the production and transportation of antimicrobial agents into the biofilm by an electrophoretic process, the genesis of additional biocide ions, or hyperoxygenation (14, 18, 19). The reduced susceptibility of biofilm bacteria to antimicrobials has been associated with localized oxygen depletion within the biofilm (1, 4). The amperage used in the experiments was sufficient for the hydrolysis of water at even the lowest levels. The production of free oxygen by electrolysis might overcome the phenomenon mentioned above. Another suggested target of the EC may be the extracellular matrix of the biofilm, which contains many types of charged particles and molecular chains with polar subsystems (3). Electric fields may influence the organization of the extracellular matrix (7). Alternative mechanisms are possible. For example, whether an antimicrobic is cationic, anionic, or uncharged may relate to its association with the bioelectric effect. Gentamicin, for example, is a cationic antimicrobic. Ciprofloxacin, gatifloxacin, and daptomycin are anionic. Vancomycin, minocycline, linezolid, trimethoprim-sulfamethoxazole, rifampin, cefazolin, and cefepime are uncharged. In this study, we have detected differences among treatments when using uncharged antimicrobials like vancomycin, an antimicrobial that has not previously been associated with the bioelectric effect (16), against S. epidermidis. Interestingly, we did not find statistically significant differences among treatments against any of the microorganisms studied when using the aminoglycoside tobramycin, the most-studied antimicrobial agent with regard to the bioelectric effect. This may be related to the strains studied or to the model design relating to EC delivery.
Several in vitro models that can be used to test the bioelectrical effect have been described; Khoury et al. used an electric modified Robbins device (14), Costerton et al. used a Perplex flow chamber to grow biofilms directly on stainless steel electrodes (9), Jass et al. used an electrical colonization cell to study biofilms suspended on one side of a dialysis membrane placed between two electrodes (12), Wellman et al. built chambers to grow and treat biofilms on polycarbonate coupons (21), Wattanakaroon and Stewart grew biofilms on polycarbonate slides in rectangular treatment chambers (20), Pickering et al. studied biofilms on the tips of stainless steel pegs (16), and Caubet et al. grew biofilms on glass slides in rectangular treatment chambers (6). We did not use the electrode itself to grow biofilms to try to avoid direct damage to the biofilms as a result of electrolysis (i.e., damage from pH changes or gas bubbles that physically push biofilms away from the electrode). This is why we developed the biofilms on the surface of a coupon and then we exposed the coupon to an electric field generated inside the experimental chamber.
The electrode composition may have an impact on the bioelectric effect; stainless steel electrodes have been most commonly studied (6, 20), but platinum and gold electrodes have also been used (14). For this study, we initially used stainless steel electrodes because stainless steel may be a component of orthopedic devices and there is some published experience with its use in studies of the bioelectric effect. However, corrosion of the electrode working as the cathode occurred, especially when 2,000 microamperes was used. Electrolysis and a pH decrease were probably the cause (18). The black-brown discoloration of the medium observed when 2,000 microamperes was passed through the medium was likely due to the release of Fe2+ and Fe3+ from the metal substratum. The corrosion issue was addressed by changing the electrode composition to graphite. We have previously demonstrated that the electrode composition (stainless steel or graphite) may play a role in the bioelectric effect observed in vitro, at least with some S. aureus strains (10). When stainless steel electrodes were used, the bioelectric effect was more pronounced against the S. aureus strains investigated than when graphite electrodes were used. Because of our usage of graphite electrodes, our results may not be as impressive as those previously reported from studies with stainless steel electrodes (i.e., a 2-log-unit reduction for P. aeruginosa and tobramycin [12, 13], a 3-log-unit reduction for P. aeruginosa and tobramycin [18], a 3-log-unit reduction for S. epidermidis or a 4-log-unit reduction for P. aeruginosa and tobramycin [14], a 4- to 5-log-unit reduction for P. aeruginosa and tobramycin [9], a 5-log-unit reduction for S. gordonii and gentamicin [20], or a 5-log-unit reduction for E. coli and tobramycin or oxytetracycline [6]). In combination with EC, we did obtain a 2.48-log-unit reduction when using tobramycin against P. aeruginosa; 2-log-unit reductions when using tobramycin, daptomycin, minocycline, or moxifloxacin against S. aureus; 2-log-unit reductions when using erythromycin, rifampin, or vancomycin against S. epidermidis; and 3-log-unit reductions when using tobramycin or minocycline against S. epidermidis. However, when we compared these log reductions with those for the antimicrobial agent-exposed coupons, we detected statistically significant differences only for vancomycin plus 2,000 microamperes EC against MRSA biofilms and for daptomycin and erythromycin plus 200 and 2,000 microamperes EC, respectively, against S. epidermidis biofilms.
Our purpose was to determine, using an in vitro model with three important bacterial biofilm pathogens and a wide range of antimicrobials, whether the bioelectric effect was generalizable and could have clinical application in biofilm-related infections. Previous studies have utilized aminoglycosides (gentamicin or tobramycin), quinolones (ciprofloxacin), or tetracycline (oxytetracycline) combined with electrical fields against P. aeruginosa, S. epidermidis, E. coli, K. pneumoniae, and S. gordonii. However, due to the limited published data, it cannot be assumed that the bioelectric effect can be generalized to all antimicrobial agents or to all bacterial species. For example, Pickering et al. (16) did not detect any significant bioelectric effect with vancomycin and S. epidermidis, and Jass and Lappin-Scott (13) showed that the bioelectric effect did not occur with piperacillin and (piperacillin-susceptible) P. aeruginosa. We show here that low-intensity EC (calculated current densities of 0.024, 0.24, and 2.4 microamperes per cm2 for 20-, 200-, and 2,000-microampere intensities, respectively) was able to enhance the antimicrobial activity of certain antimicrobial agents against bacterial biofilms (i.e., vancomycin against MRSA and daptomycin or erythromycin against S. epidermidis). The phenomenon observed in our study was less pronounced than that observed and reported in other studies (6, 9, 12, 13, 14, 18, 20, 21). However, these data are more comprehensive in terms of the number of antimicrobial agents and bacterial species studied under the same conditions. In addition, this is the first study to test the hypothesis that the in vitro application of EC may increase the antimicrobial killing of MRSA biofilms.
There are limitations of our study. We recognize that single isolates do not necessarily represent all members of the species studied. Also, our model did not incorporate a conditioning film of proteins on the coupons such as one that might be present on biofilms in vivo. Nevertheless, our wide range of exploratory experiments in which antimicrobials of several classes were used to treat biofilms of three different bacterial species indicate that the enhancement of the efficacy of these agents by EC is not a generalizable phenomenon.
An obvious human application of the bioelectric effect is in the management of infections associated with orthopedic hardware. EC has been used in experimental models to drive chemotherapeutic molecules into solid tumors (17) and antimicrobial agents into the inner ear (9). Direct EC has already been safely used in humans for the healing of fractures (5). Ideally, if the bioelectric effect is applied to human infections, EC should be delivered in a noninvasive (e.g., transcutaneous) or minimally invasive (e.g., subcutaneous) fashion. The attachment of wires directly to the surfaces of foreign bodies is not ideal since the wires themselves may be a conduit for microorganisms. The application of EC with antimicrobial chemotherapy in humans may have the potential to eliminate the need for device removal in human device-related infections. The best treatment combinations obtained in our studies should be tested in an experimental animal model. More studies are warranted to address the mechanisms of electrical enhancement of antimicrobial activity against bacterial biofilms.
FIG. 3.
Mean LRFs and standard deviations for Pseudomonas aeruginosa after exposure to four different treatments (i.e., antimicrobial agent alone, antimicrobial agent plus 20 microamperes EC, antimicrobial agent plus 200 microamperes EC, and antimicrobial agent plus 2,000 microamperes EC).
FIG. 4.
Mean LRFs and standard deviations for Staphylococcus aureus after exposure to four different treatments (i.e., antimicrobial agent alone, antimicrobial agent plus 20 microamperes EC, antimicrobial agent plus 200 microamperes EC, and antimicrobial agent plus 2,000 microamperes EC).
Acknowledgments
This work was supported by the National Institutes of Health (grant R21AI061407).
We have no disclosures or conflicts of interest to report.
We thank Xenogen Corp. for providing strains Xen 30, Xen 43, and Xen 5 and April Horne from the Mayo Division of Engineering for designing and fabricating the current generator/controllers and test chambers. We thank Gary Nguyen and Emily McLean for assistance with the experiments.
Footnotes
Published ahead of print on 25 August 2008.
REFERENCES
- 1.Anderl, J. N., J. Zahller, F. Roe, and P. S. Stewart. 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 47:1251-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anwar, H., M. K. Dasgupta, and J. W. Costerton. 1990. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother. 34:2043-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blenkinsopp, S. A., A. E. Khoury, and J. W. Costerton. 1992. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 58:3770-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borriello, G., E. Werner, F. Roe, A. M. Kim, G. D. Ehrlich, and P. S. Stewart. 2004. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 48:2659-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brighton, C. T., Z. B. Friedenberg, E. I. Mitchell, and R. E. Booth. 1977. Treatment of nonunion with constant direct current. Clin. Orthop. Relat. Res. 124:106-123. [PubMed] [Google Scholar]
- 6.Caubet, R., F. Pedarros-Caubet, M. Chu, E. Freye, M. de Belem Rodrigues, J. M. Moreau, and W. J. Ellison. 2004. A radio-frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob. Agents Chemother. 48:4662-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cevc, G. 1990. Membrane electrostatics. Biochim. Biophys. Acta 1031:311-382. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., B. Ellis, K. Lam, F. Johnson, and A. E. Khoury. 1994. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 38:2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Pozo, J. L., M. S. Rouse, M. Fernandez Sampedro, J. M. Steckelberg, and R. Patel. 2007. Electrode composition and electrical enhancement of rifampin activity against methicillin resistant Staphylococcus aureus (MRSA) biofilms, abstr. B73. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 11.del Pozo, J. L., and R. Patel. 2007. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 82:204-209. [DOI] [PubMed] [Google Scholar]
- 12.Jass, J., J. W. Costerton, and H. M. Lappin-Scott. 1995. The effect of electrical currents and tobramycin on Pseudomonas aeruginosa biofilms. J. Ind. Microbiol. 15:234-242. [DOI] [PubMed] [Google Scholar]
- 13.Jass, J., and H. M. Lappin-Scott. 1996. The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms. J. Antimicrob. Chemother. 38:987-1000. [DOI] [PubMed] [Google Scholar]
- 14.Khoury, A. E., K. Lam, B. Ellis, and J. W. Costerton. 1992. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 38:M174-M178. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickering, S. A., R. Bayston, and B. E. Scammell. 2003. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J. Bone Joint Surg. Br. 85:588-593. [DOI] [PubMed] [Google Scholar]
- 17.Sersa, G., and D. Miklavcic. 1990. Inhibition of SA-1 tumor growth in mice by human leukocyte interferon alpha combined with low-level direct current. Mol. Biother. 2:165-168. [PubMed] [Google Scholar]
- 18.Stewart, P. S., W. Wattanakaroon, L. Goodrum, S. M. Fortun, and B. R. McLeod. 1999. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 43:292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoodley, P., D. deBeer, and H. M. Lappin-Scott. 1997. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob. Agents Chemother. 41:1876-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wattanakaroon, W., and P. S. Stewart. 2000. Electrical enhancement of Streptococcus gordonii biofilm killing by gentamicin. Arch. Oral Biol. 45:167-171. [DOI] [PubMed] [Google Scholar]
- 21.Wellman, N., S. M. Fortun, and B. R. McLeod. 1996. Bacterial biofilms and the bioelectric effect. Antimicrob. Agents Chemother. 40:2012-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]