Abstract
This study describes an in vivo model for evaluating the sterilizing activity of compounds against persisting Mycobacterium tuberculosis. The initial treatment with isoniazid and rifampin in granulocyte-macrophage colony-stimulating factor gene-disrupted mice reduced the number of bacteria more than 99% within 3 weeks. A subsequent treatment with individual drugs was performed to assess their activity on the 1% of remaining bacilli and disease relapse.
One of the challenges to develop new antituberculosis (anti-TB) drugs is the ability of bacilli to persist for long periods of time in the host. The bacilli's slow replication rate, phenotypic drug tolerance (1, 9, 15, 28), and ability to enter into a less metabolically active state due to their environment (26) are thought to be some of the reasons that drug treatment currently takes 6 to 9 months to complete. The killing kinetics of drugs against Mycobacterium tuberculosis bacilli are biphasic in both in vitro and in vivo studies. The initial killing of the bacilli is rapid, resulting in vitro in the death of the majority of organisms (90 to 99%), while the remaining populations of bacteria are far less responsive to the drug effect. In mice, 99% of the bacteria in the lung are killed within 2 to 3 weeks of drug treatment, while it requires at least 3 more months of treatment to clear the remaining 1% of bacteria (7, 12, 20, 25). This phenomenon of a decrease in the bactericidal activities of drugs has been observed for a variety of species of bacteria and for many drug classes tested (1, 24, 28), and for the purpose of this paper, we will define it here as drug tolerance. The mechanisms of phenotypic tolerance in TB are currently unknown (3, 25). Because current anti-TB drugs target mainly the actively replicating bacterial population, there is a great need to develop drugs with activity against these drug-tolerant, persisting bacilli.
Mouse models have been used extensively to evaluate anti-TB drugs in vivo (8, 12, 14). Most mouse models are used to evaluate the activity of compounds against a combination of different bacterial populations (e.g., actively replicating, slowly replicating, and drug-tolerant bacilli). The most widely used mouse model which focuses on persistent TB is the so-called Cornell model (16-19). In this model developed by McCune et al. (16-19), mice receive TB treatment until no bacteria can be cultured from any organ. After the cessation of treatment, however, the mice have the potential for a relapse infection, and the bacteria from the lungs and spleens can be cultured again. This model has been widely used for evaluating new vaccine and drug candidates for sterilizing activity in vivo (13, 14). There are, however, significant limitations to the Cornell model (10, 23). The first limitation is that drug treatments last for 3 to 6 months, making the model time consuming and labor intensive. Second, the relapse of infection after treatment is totally unpredictable, and therefore, very large numbers of animals are required to reach sufficient statistical power (10). In this study, we propose a new mouse model which not only decreases the length of the treatments significantly but also has less variability and shows an immediate relapse of infection in all animals carrying bacilli.
The mouse model is based on the use of granulocyte-macrophage colony-stimulating factor gene-disrupted mice (GM-CSF−/−) for evaluating drugs for efficacy against drug-tolerant bacteria persisting after initial treatment. These mice are unable to generate protective cytokines, resulting in a rapid growth of bacilli as well as a rapid relapse of infection after the cessation of drug treatment. (4, 6). Briefly, 2- to 8-month-old GM-CSF−/− C57BL/6 mice (4) were exposed to a low-dose aerosol (LDA) infection with the M. tuberculosis Erdman strain (TMCC 107) by a Glas-Col inhalation exposure system as previously described (8, 11). Frozen stocks of M. tuberculosis were used for all experiments to increase the reproducibility of the data. The described experiment is representative of four experiments which all gave similar results. Animals were sacrificed 1 day and 2 weeks after LDA infection by CO2 inhalation to determine the bacterial loads prior to drug treatment. Homogenates from the lungs and spleens were plated as described before (12). A schematic overview of the treatment regimens is depicted in Fig. 1. The initial drug treatment with isoniazid (INH) and rifampin (RIF) started 2 weeks after infection and lasted for 3 weeks, reducing the bacterial load by more than 99% after 3 weeks (in all four experiments performed). The drugs were administered in the drinking water: INH (Sigma Aldrich) at 100 mg/liter and RIF (Sigma Aldrich) first dissolved in 100% dimethyl sulfoxide and subsequently diluted in water to 100 mg/liter (final dimethyl sulfoxide concentration, 0.5%). The preliminary experiments showed that this concentration was sufficient to provide a daily dose of 25 mg/kg for each drug. After 3 weeks of treatment, some of the animals were sacrificed to determine their bacterial loads. The remaining animals were randomized into treatment groups of five to six mice each. Subsequent treatment with the following single drugs lasted for 4 weeks: INH, RIF, ethambutol (EMB; Sigma-Aldrich), or moxifloxacin (MXF; kindly provided by the Southern Research Institute [SRI], Birmingham, AL). The drugs were dissolved in water and administered in the following doses: EMB at 150 mg/kg, INH at 25 mg/kg, MXF at 300 mg/kg, and RIF at 20 mg/kg. These are dosages which have been shown to be equivalent to those used in the clinic for TB patients (5, 21, 29). The drugs were administered by oral gavage 5 days per week. After 4 weeks of treatment with the single drugs, animals from each treatment group were sacrificed. After an additional 4 weeks without further treatment, parallel groups of the remaining animals in each drug group were sacrificed and the lungs and spleens plated for bacterial enumeration. The colonies were counted after incubation for 4 to 6 weeks at 37°C. A statistical analysis was performed using one-way analysis of variance, followed by a multiple comparison analysis of variance by the Newman-Keuls test using GraphPad Prism v4.02 (GraphPad, San Diego, CA).
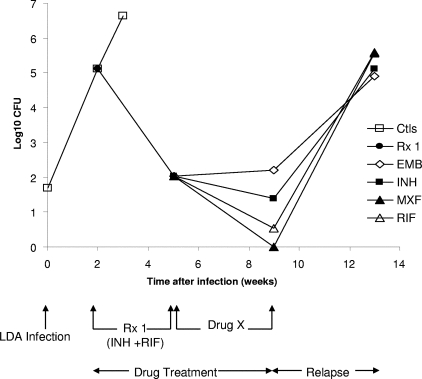
FIG. 1.
Numbers of viable M. tuberculosis organisms in the lungs of infected GM-CSF−/− mice. Two weeks after LDA infection with M. tuberculosis, the mice were treated for 3 weeks with INH plus RIF in drinking water. Subsequently, individual drugs were administered by gavage for an additional 4 weeks. Data points represent the mean log10 viable bacilli present in whole lung homogenates. Ctls, untreated controls; Rx 1, INH plus RIF treatment; Drug X, subsequent treatment with single drugs for 4 weeks.
The results of this study showed for the untreated control group a bacterial load in the lungs of 5.12 log10 CFU at 2 weeks after LDA infection. The results are shown in Fig. 1 and Table 1. At 2 weeks after LDA infection, INH and RIF treatment was initiated in the drinking water for 3 weeks, which reduced the bacterial load over 99% (to 2.03 log10 CFU). After the mice were subsequently treated for 4 weeks with individual drugs, the results showed MXF to be the most-effective drug, with no mice showing any bacterial growth in the lungs. In the RIF-treated group, one out of five mice had only a few bacteria (0.54 log10 CFU). In the INH-treated group, the mice showed an average bacterial load of 1.39 log10 CFU, which was similar to the average of 2.20 log10 CFU (P > 0.05) for the EMB-treated group. MXF was significantly more active in the lungs than INH as well as EMB in reducing the bacterial load (P < 0.05), and the activity of RIF was found to be significantly better than that of EMB (P < 0.05). In the spleens, the bacterial loads were already cleared after the initial treatment with INH and RIF. After the cessation of the drug treatment and the resting of the animals for 4 weeks, bacterial regrowth was observed in all animals in every treatment group in both the lungs and the spleens.
TABLE 1.
Number of viable M. tuberculosis organisms in the lungs and spleens of infected GM-CSF−/− mice after the initial INH plus RIF treatment and the subsequent treatment with individual drugsa
| Group | Lungs
|
Spleens
|
||||
|---|---|---|---|---|---|---|
| Log10 CFU | SEM | n | Log10 CFU | SEM | n | |
| 2 wk after infection | 5.12 | 0.33 | 5/5 | 1.73 | 0.39 | 4/5 |
| 3 wk INH/RIF | 2.03 | 0.4 | 5/5 | 0 | 0 | 0/5 |
| 3 wk INH/RIF + 4 wk EMB | 2.2 | 0.17 | 5/5 | 0 | 0 | 0/5 |
| 3 wk INH/RIF + 4 wk INH | 1.39 | 0.52 | 5/6 | 0.23 | 0.23 | 1/6 |
| 3 wk INH/RIF + 4 wk MXF | 0 | 0 | 0/5 | 0 | 0 | 0/5 |
| 3 wk INH/RIF + 4 wk RIF | 0.54 | 0.54 | 1/5 | 0 | 0 | 0/5 |
| 3 wk INH/RIF + 4 wk EMB + 4 wk rest | 4.9 | 0.24 | 5/5 | 1.84 | 0.52 | 4/5 |
| 3 wk INH/RIF + 4 wk INH + 4 wk rest | 5.11 | 0.18 | 6/6 | 2.78 | 0.26 | 6/6 |
| 3 wk INH/RIF + 4 wk MXF + 4 wk rest | 5.56 | 0.84 | 5/5 | 2.82 | 0.78 | 4/5 |
| 3 wk INH/RIF + 4 wk RIF + 4 wk rest | 5.58 | 0.28 | 5/5 | 3.28 | 0.37 | 4/5 |
Two weeks after LDA infection with M. tuberculosis, mice were treated for 3 weeks with INH plus RIF in the drinking water. Subsequently, individual drugs were administered by gavage for an additional 4 weeks. The mice were then rested without treatment for an additional 4 weeks. n, the number of animals carrying culturable CFU/total number of animals in the group; SEM, standard error of the mean.
The obtained results for the lungs are consistent with earlier data by others describing the potent sterilizing activity of MXF in vitro (20) as well as in in vivo long-term mouse models (22, 29). In earlier studies, RIF also has shown sterilizing activity in vitro (12, 27) as well as good sterilizing activity in vivo (2). The fact that INH and EMB showed less activity in this model was as expected, as cell-wall inhibitors have activity primarily against actively replicating bacteria (20). All mice showed relapses of infection, which means that treatment with more-potent drugs, for instance, TMC207, could be tested in this model. The main advantage of this model is to be able to rank new lead compounds for their potential to sterilize drug-tolerant bacilli prior to initiating long-term drug combination trials in wild-type mice. This would be a rapid way to collect additional data to prioritize leads in the TB drug development process.
An analysis of the sterilizing potential and the relapse of infection is an important component of clinical trials evaluating drug regimens used to treat TB. The classical Cornell model attempts to address persisting bacteria and subsequent relapse frequencies, but this model is lengthy and unpredictable and has problems with statistical power (10, 23). We present here a new model using GM-CSF gene-disrupted mice in which relapse is immediate and reproducible. New drugs can be compared for their abilities to reduce the number of bacteria persisting after initial treatment. Hence, this new model could be used to select for new experimental compounds specifically targeting these persisting bacteria, the elimination of which would shorten the duration of therapy.
Acknowledgments
We acknowledge the staff of the Laboratory Animal Resources (Colorado State University [CSU]) for animal care.
Support was provided by NIH contracts NO1 AI-95385 at CSU and N01-AI-95364 at the Southern Research Institute, Birmingham, AL.
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Balaban, N. Q., J. Merrin, R. Chait, L. Kowalik, and S. Leibler. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622-1625. [DOI] [PubMed] [Google Scholar]
- 2.Batten, J. 1970. Rifampicin in the treatment of experimental tuberculosis in mice: sterilization of tubercle bacilli in the tissues. Tubercle 51:95-99. [DOI] [PubMed] [Google Scholar]
- 3.Dhar, N., and J. D. McKinney. 2007. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr. Opin. Microbiol. 10:30-38. [DOI] [PubMed] [Google Scholar]
- 4.Dranoff, G., A. D. Crawford, M. Sadelain, B. Ream, A. Rashid, R. T. Bronson, G. R. Dickersin, C. J. Bachurski, E. L. Mark, J. A. Whitsett, et al. 1994. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264:713-716. [DOI] [PubMed] [Google Scholar]
- 5.Ellner, J., P. Brennan, and D. E. Young. 2001. Scientific blueprint for TB drug development. Tuberculosis (Edinburgh) 81:1-52. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Juarrero, M., J. M. Hattle, A. Izzo, A. P. Junqueira-Kipnis, T. S. Shim, B. C. Trapnell, A. M. Cooper, and I. M. Orme. 2005. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J. Leukoc. Biol. 77:914-922. [DOI] [PubMed] [Google Scholar]
- 7.Grosset, J. 1978. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Tubercle 59:287-297. [PubMed] [Google Scholar]
- 8.Kelly, B. P., S. K. Furney, M. T. Jessen, and I. M. Orme. 1996. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2809-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kussell, E., R. Kishony, N. Q. Balaban, and S. Leibler. 2005. Bacterial persistence: a model of survival in changing environments. Genetics 169:1807-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenaerts, A. J., P. L. Chapman, and I. M. Orme. 2004. Statistical limitations to the Cornell model of latent tuberculosis infection for the study of relapse rates. Tuberculosis (Edinburgh) 84:361-364. [DOI] [PubMed] [Google Scholar]
- 11.Lenaerts, A. J., V. Gruppo, J. V. Brooks, and I. M. Orme. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenaerts, A. M., S. E. Chase, A. J. Chmielewski, and M. H. Cynamon. 1999. Evaluation of rifapentine in long-term treatment regimens for tuberculosis in mice. Antimicrob. Agents Chemother. 43:2356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenaerts, A. M., S. E. Chase, and M. H. Cynamon. 2000. Evaluation of rifalazil in a combination treatment regimen as an alternative to isoniazid-rifampin therapy in a mouse tuberculosis model. Antimicrob. Agents Chemother. 44:3167-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin, B. R. 2004. Microbiology. Noninherited resistance to antibiotics. Science 305:1578-1579. [DOI] [PubMed] [Google Scholar]
- 16.McCune, R. J., W. McDermott, and R. Tompsett. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J. Exp. Med. 104:763-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCune, R. J., and R. Tompsett. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J. Exp. Med. 104:737-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCune, R. M., F. M. Feldmann, H. P. Lambert, and W. McDermott. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J. Exp. Med. 123:445-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCune, R. M., F. M. Feldmann, and W. McDermott. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J. Exp. Med. 123:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchison, D. A. 2004. The search for new sterilizing anti-tuberculosis drugs. Front. Biosci. 9:1059-1072. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki, E., M. Miyazaki, J. M. Chen, R. E. Chaisson, and W. R. Bishai. 1999. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 43:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, R. J. O'Brien, A. N. Vernon, R. E. Chaisson, W. R. Bishai, and J. H. Grosset. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 23.Scanga, C. 1999. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect. Immun. 67:4531-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuomanen, E. 1986. Phenotypic tolerance: the search for beta-lactam antibiotics that kill nongrowing bacteria. Rev. Infect. Dis. 8(Suppl. 3):S279-S291. [DOI] [PubMed] [Google Scholar]
- 25.Wallis, R. S., S. Patil, S. H. Cheon, K. Edmonds, M. Phillips, M. D. Perkins, M. Joloba, A. Namale, J. L. Johnson, L. Teixeira, R. Dietze, S. Siddiqi, R. D. Mugerwa, K. Eisenach, and J. J. Ellner. 1999. Drug tolerance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 27.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiuff, C., R. M. Zappala, R. R. Regoes, K. N. Garner, F. Baquero, and B. R. Levin. 2005. Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations. Antimicrob. Agents Chemother. 49:1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimatsu, T., E. Nuermberger, S. Tyagi, R. Chaisson, W. Bishai, and J. Grosset. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]