Abstract
There are currently no standardized phenotypic methods for the screening and detection of AmpC enzymes. This study aimed to evaluate different methods to detect AmpC enzymes in Escherichia coli, Klebsiella spp., and Proteus spp., comparing the results from two disk-based methods and an agar dilution method. AmpC activity was determined for 255 clinical isolates by use of a three-dimensional enzyme assay combined with a multiplex PCR assay for plasmid-borne ampC genes. These results were compared against a disk-based inhibitor assay using various combinations of cefpodoxime and cefoxitin as antibiotic substrates and boronic acid or cloxacillin as an AmpC inhibitor. The presence of enzyme induction by disk approximation was evaluated using imipenem, cefoxitin, and amoxicillin-clavulanate as inducing agents against ceftazidime. Finally, an agar dilution assay was performed, using cefoxitin with and without added cloxacillin. AmpC activity was present in 49.8% of test isolates, 93.7% of which were positive for plasmid-borne ampC genes. CIT-like enzymes were predominant in E. coli, and DHA-like enzymes were predominant in Klebsiella spp. The disk-based inhibitor tests performed better than the agar dilution assay, while detection of AmpC by disk induction had a poor sensitivity. The cefoxitin-cloxacillin disk combination provided the best overall performance, with a sensitivity and specificity of 95%. This study confirmed the accuracy of disk-based inhibitor screening for AmpC enzymes, which proved reliable at detecting CIT- and DHA-like plasmid-borne ampC genes. The methods are simple enough for introduction into clinical microbiology laboratories.
Resistance to expanded-spectrum cephalosporins in Enterobacter spp., Citrobacter spp., and Serratia spp. may develop through the expression of chromosomally encoded class C beta-lactamases, also known as AmpC beta-lactamases. Although a chromosomal ampC gene is present in Escherichia coli, it is not usually expressed because of the presence of a transcriptional attenuator coupled with a weak promoter (12, 23). The transfer of ampC genes to plasmids has resulted in their dissemination among Enterobacteriaceae, with the consequence that ampC-encoded beta-lactamases are now present in strains of Klebsiella spp., E. coli, Proteus mirabilis, and Salmonella spp. Plasmid-mediated AmpC enzymes have been described from diverse geographic areas, including the United Kingdom, the United States, and Asia (7, 8, 11, 18, 24).
The capability to detect AmpC is important to improve the clinical management of infections and provide sound epidemiological data, but at present, there are no standardized phenotypic screening methods that are readily available to microbiology laboratories. Reduced susceptibility to cefoxitin in the Enterobacteriaceae may be an indicator of AmpC activity, but cefoxitin resistance may also be mediated by alterations to outer membrane permeability (6). Conventional susceptibility testing may also fail to reliably detect these strains, as the MICs of third-generation cephalosporins may fall below the currently recommended breakpoints from the CLSI (5, 22). Enzyme extraction methods have traditionally been cited as the optimum phenotypic detection method for AmpC activity. However, these are labor-intensive and not suitable for routine clinical use. Inhibitors of the AmpC enzyme are well described and include boronic acid compounds, cloxacillin, and novel inhibitors such as Syn2190. Minimum inhibitory testing assays incorporating these inhibitors have been described (1, 5), analogous to the use of beta-lactamase inhibitor combinations for detection of extended-spectrum beta-lactamases. These inhibitors have also been incorporated into disk-based assays, using a variety of combinations of antibiotic substrates and inhibitors (2, 5). The use of disk approximation tests by Kirby-Bauer testing to detect inducible AmpC activity has also been described, using one antibiotic as an inducing substrate and a second antibiotic as a reporter substrate (19).
At present, phenotypic tests are not able to differentiate between chromosomal ampC genes and ampC genes that are carried on plasmids. Perez-Perez and Hanson described a multiplex PCR for six families of plasmid-carried ampC genes (some originating from Hafnei alvei, Citrobacter freundii, Morganella morganii, and Enterobacter cloacae), which may be used to detect the presence of these externally acquired ampC genes in E. coli, Klebsiella spp., and Proteus spp. (17).
Clinical laboratories commonly use either dilution or disk-based methods for susceptibility testing. This study set out to evaluate the accuracy of a variety of phenotypic screening methods based on these commonly used methods for the detection of AmpC in clinical isolates of E. coli, Klebsiella spp., and Proteus mirabilis.
MATERIALS AND METHODS
A total of 255 clinical isolates of E. coli (n = 174), Klebsiella spp. (n = 76), and Proteus mirabilis. (5) were collected from two geographically separate hospitals in Singapore from 2005 to 2007. Strains were predominantly cultured from urine (n = 218), blood (n = 15), fluid and tissue samples (n = 11), and other superficial body sites (n = 11).
All isolates were tested for the phenotypic presence of AmpC activity by using enzyme extraction methods as described by Nasim and colleagues (14). In brief, organisms were inoculated into 10 ml of Trypticase soy broth and incubated at 35°C for 4 h. Crude enzyme extract solutions, obtained by subjecting the centrifuged bacterial suspension to four freeze-thaw cycles, were inoculated into prepared wells in cefoxitin-agar medium containing 4 μg/ml of cefoxitin. A zone of growth around the periphery of a test well was interpreted as demonstrating the presence of AmpC activity. All isolates were also tested using the multiplex PCR assay described by Perez-Perez and Hanson (17), which detects the presence of five families of plasmid-mediated ampC genes. Since E. coli strains with derepressed chromosomal ampC are not detected by the PCR assay, the gold standard for detection of AmpC activity was taken to be a combination of the phenotypic and genotypic testing results.
AmpC screening using dilution methods.
MICs of cefoxitin were determined for all study isolates by agar dilution following CLSI methods (4). Doubling dilutions of cefoxitin were prepared in Mueller-Hinton agar (Becton Dickinson), with and without the addition of a fixed concentration of cloxacillin (100 μg/ml), to provide twofold concentrations ranging from 0.125 μg/ml to 256 μg/ml. Isolates with a ≥4-fold reduction in cefoxitin MIC in the presence of cloxacillin were considered to be positive for AmpC.
AmpC screening using disk diffusion.
The effectiveness of AmpC screening using 30-μg cefoxitin (Becton Dickinson) and 10-μg cefpodoxime (Oxoid, United Kingdom) disks combined with a variety of AmpC inhibitor compounds was evaluated. The added concentration of the supplemental inhibitor compounds was derived from previous experiments with strains of Enterobacteriaceae (both ampC-positive and ampC-negative isolates). The selected concentration of inhibitor was shown to obtain clear zones of inhibition for ampC-positive members of the Enterobacteriaceae while not appearing to inhibit the growth of ampC-negative strains (data not shown).
The 30-μg cefoxitin disk was supplemented with either 400 μg of phenylboronic acid (P20009; Sigma-Aldrich, Singapore), 200 μg of cloxacillin (Sigma-Aldrich, Singapore), or both compounds. The 10-μg cefpodoxime disks were supplemented with either 400 μg of phenylboronic acid or 200 μg of cloxacillin. An organism that demonstrated a defined increase in zone diameter around the antibiotic disk with added inhibitor compound compared to that with the antibiotic-containing disk alone was considered to be an AmpC producer.
The use of disk approximation techniques to detect inducible AmpC activity was also tested, using 10-μg imipenem, 30-μg cefoxitin, and 20/10-μg amoxicillin-clavulanate disks as the inducing substrates and 30-μg ceftazidime disks (all from Bio-Rad, France) as the reporter substrate. Disks were applied by use of an applicator at a distance of 20 mm, and any obvious blunting or flattening of the zone of inhibition between the ceftazidime disk and the inducing substrates was interpreted as a positive result for AmpC.
All susceptibility testing was performed using 0.5-McFarland-standard suspensions prepared from overnight cultures, applied to Mueller-Hinton agar (Becton Dickinson), and incubated at 35°C for 16 to 18 h. Zones of inhibition were measured to the edge of obvious inhibition, ignoring any microcolonies present within a clear zone of inhibition.
Evaluation of shelf life of prepared disks.
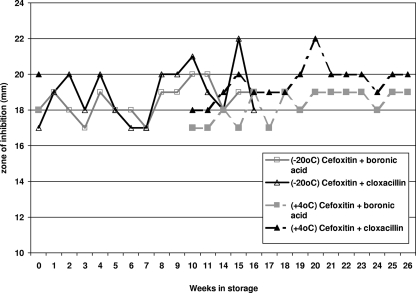
In order to evaluate the possible effects of storage time and conditions on disks containing inhibitor compounds, a batch of 30-μg cefoxitin disks from a single lot was impregnated with boronic acid or cloxacillin, as described above. The disks were initially tested on a single E. coli strain carrying a CIT-type enzyme, and the zones of inhibition were recorded. The prepared disks were then stored at −20°C and +4°C, and repeat testing using the same control strain was carried out at weekly intervals, from weeks 1 to 15 for the disks stored at −20°C and from weeks 10 to 26 for the disks stored at +4°C.
RESULTS
AmpC activity was present in 127 (49.8%) of the test isolates, 119 of which were positive by multiplex PCR for plasmid-mediated ampC genes. The majority of ampC-positive strains were isolated from urine cultures (n = 99), but there were also ampC-positive isolates obtained from all other sample types. Enzymes from the CIT group (CMY-like enzymes derived from C. freundii) were predominantly present in E. coli (n = 67) but were also found in Klebsiella spp. (n = 3) and Proteus mirabilis (n = 4). DHA-like enzymes (originally derived from Morganella morganii) were present predominantly in Klebsiella spp. (n = 41) but also in a minority of E. coli isolates (n = 4). Non-plasmid-derived AmpC activity was present in eight strains of E. coli and was presumed to originate from hyperproduction of endogenous AmpC enzyme (Table 1). No enzymes belonging to the ACC, FOX, MOX, or EBC family were detected.
TABLE 1.
Distribution of ampC genes within study isolates
| Organism | No. (%) of isolates
|
|||
|---|---|---|---|---|
| ampC negative | DHA positive | CIT positive | Endogenous ampC positivea | |
| E. coli | 95 (55) | 4 (2) | 67 (39) | 8 (5) |
| Klebsiella spp. | 32 (42) | 41 (54) | 3 (4) | NA |
| Proteus mirabilis | 0 (0) | 0 (0) | 4 (100) | NA |
NA, not applicable.
A total of 113 (90.0%) of the AmpC-positive strains were detected by agar dilution, while 13 AmpC-negative strains were positive by this method. The overall sensitivity and specificity of detecting AmpC enzymes by cefoxitin agar dilution were 89.0% and 90.0%, respectively.
Detection of AmpC enzymes by disk approximation showed poor results, with only 32 (25.2%) of the AmpC-positive isolates showing blunting of the ceftazidime zone. For AmpC isolates detected by this method, imipenem was the strongest inducing antibiotic. Disk approximation showed a sensitivity of 25.2% and a specificity of 99.2%.
Zones of inhibition were plotted for the cefoxitin and cefpodoxime disks, together with the zones of inhibition for the corresponding antibiotic disks with added AmpC inhibitors. The sensitivity and specificity for AmpC detection for each antibiotic-inhibitor combination were calculated by applying cutoffs (calculated from the difference in zone diameter between the antibiotic disk and the antibiotic disk with added inhibitor) of ≥4 mm, ≥5 mm, and ≥6 mm. The best test sensitivity (96%) was obtained with cefoxitin with added cloxacillin and boronic acid, using an increase in zone diameter of ≥4 mm as the cutoff. However, there was a corresponding fall in test specificity (81%). The best test specificity (99%) was obtained with the cefoxitin-cloxacillin combination, using a cutoff of ≥6 mm, but the test sensitivity was low (88%). Overall, the best sensitivity (95%) and specificity (95%) were obtained using a combination of cefoxitin and cloxacillin and applying a cutoff of an increase in zone diameter of ≥4 mm. The results for all antibiotic-inhibitor combinations are shown in Table 2.
TABLE 2.
Performance of antibiotic disks with added inhibitor substrates
| Antibiotic | Inhibitor substrate | Cutoff at increase of zone diam of:
|
|||||
|---|---|---|---|---|---|---|---|
| ≥4 mm
|
≥5 mm
|
≥6 mm
|
|||||
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | ||
| Cefoxitin | Boronic acid | 94 | 89 | 91 | 95 | 85 | 98 |
| Cloxacillin | 95 | 95 | 92 | 98 | 88 | 99 | |
| Boronic acid plus cloxacillin | 96 | 81 | 95 | 94 | 94 | 94 | |
| Cefpodoxime | Boronic acid | 78 | 91 | 75 | 95 | 73 | 97 |
| Cloxacillin | 83 | 92 | 77 | 97 | 72 | 97 | |
Results for the zones of inhibition obtained by weekly testing of the stored antibiotic-inhibitor disk combinations were plotted against time in weeks. There was no decrease in the zone of inhibition obtained for the prepared disks even after storage for 16 weeks at −20°C and 26 weeks at +4°C (Fig. 1).
FIG. 1.
Zones of inhibition for antibiotic disk-inhibitor combinations following storage, as tested on a strain of E. coli carrying the CMY gene.
DISCUSSION
To our knowledge, this is the first study to evaluate the accuracy of various phenotypic techniques used to screen for AmpC activity, using a large number of bacterial isolates. We chose simple methods that used readily available materials. Various modifications of tests derived from enzyme extraction methods have been reported in the literature (21), but in general, these are too time-consuming or laborious for routine use. Novel inhibitors of the AmpC enzyme have also been described (15), but some of these may not be readily available.
The detection of AmpC enzymes by the presence of inducible distortion around ceftazidime disks lacked sensitivity. Agar dilution proved better, but the best results were obtained by screening using antibiotic disks impregnated with AmpC inhibitor compounds. However, the results obtained varied according to the antibiotic substrate and inhibitor compound used. Using cefoxitin as the antibiotic substrate clearly provided better results than those obtained by using cefpodoxime. The best overall results were obtained by using a cefoxitin disk with added cloxacillin and applying the interpretative criterion of an increase in the zone size of ≥4 mm for the disk with added inhibitor compared to that for the antibiotic disk alone. To further facilitate the routine implementation of a disk-based screening assay, prepared disks may be stored at +4°C or −20°C for at least 16 weeks without a loss of potency.
This study has some limitations. Only DHA-like and CIT-like enzymes were tested and evaluated in this study, as no plasmid-mediated enzymes from the other families were present in the study isolates. In particular, the ACC-1 enzyme appears to be inhibited by cefoxitin (20). E. coli and Klebsiella sp. isolates with the ACC enzyme are susceptible to cefoxitin in vitro (13), and one other study reported the failure of cefoxitin disk-based screening methods to detect this particular enzyme (2). CIT-type (and, to a lesser extent, DHA-type) enzymes appear to be prevalent in Asia and Canada (7, 9, 10, 18), while a minority of ACC-type enzymes were reported from the United Kingdom (24). There are fewer published data from the United States, but one study reported that FOX-type enzymes were predominantly present in Enterobacteriaceae with plasmid-mediated enzymes (11). Further validation of the study methods in different geographic regions may be warranted.
In conclusion, this report evaluated several methods of screening for AmpC enzymes and demonstrated that using a cefoxitin-cloxacillin disk combination accurately detects the majority of AmpC-positive isolates. The importance of detecting AmpC-producing isolates is highlighted by data showing high clinical failure rates when AmpC-producing strains of Klebsiella pneumoniae are treated with cephalosporin agents (16) or the subsequent development of antibiotic resistance in such strains (3).
Acknowledgments
This study was funded in part by a grant from the National Medical Research Council.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Babini, G. S., and D. M. Livermore. 2000. Antimicrobial resistance amongst Klebsiella spp. collected from intensive care units in Southern and Western Europe in 1997-1998. J. Antimicrob. Chemother. 45:183-189. [DOI] [PubMed] [Google Scholar]
- 2.Brenwald, N. P., G. Jevons, J. Andrews, L. Ang, and A. P. Fraise. 2005. Disc methods for detecting AmpC β-lactamase-producing clinical isolates of Escherichia coli and Klebsiella pneumoniae. J. Antimicrob. Chemother. 56:600-601. [DOI] [PubMed] [Google Scholar]
- 3.Choi, S. H., J. E. Lee, S. J. Park, S. O. Lee, J. Y. Jeong, M. N. Kim, J. H. Woo, and Y. S. Kim. 2008. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrob. Agents Chemother. 52:995-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI document M7-A7, vol. 26. Clinical Laboratory Standards Institute, Wayne, PA.
- 5.Coudron, P. E. 2005. Inhibitor-based methods for detection of plasmid-mediated AmpC beta-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J. Clin. Microbiol. 43:4163-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Alles, S., M. Conejo, A. Pascual, J. M. Tomas, V. J. Benedi, and L. Martinez-Martinez. 2000. Relationship between outer membrane alterations and susceptibility to antimicrobial agents in isogenic strains of Klebsiella pneumoniae. J. Antimicrob. Chemother. 46:273-277. [DOI] [PubMed] [Google Scholar]
- 7.Koh, T. H., L. H. Sng, G. Wang, L. Y. Hsu, R. T. Lin, and N. W. Tee. 2007. Emerging problems with plasmid-mediated DHA and CMY AmpC beta-lactamases in Enterobacteriaceae in Singapore. Int. J. Antimicrob. Agents 30:278-280. [DOI] [PubMed] [Google Scholar]
- 8.Lee, K., S. G. Hong, Y. J. Park, H. S. Lee, W. Song, J. Jeong, D. Yong, and Y. Chong. 2005. Evaluation of phenotypic screening methods for detecting plasmid-mediated AmpC beta-lactamases-producing isolates of Escherichia coli and Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 53:319-323. [DOI] [PubMed] [Google Scholar]
- 9.Lee, K., M. Lee, J. H. Shin, M. H. Lee, S. H. Kang, A. J. Park, D. Yong, and Y. Chong. 2006. Prevalence of plasmid-mediated AmpC beta-lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb. Drug Resist. 12:44-49. [DOI] [PubMed] [Google Scholar]
- 10.Li, Y., Q. Li, Y. Du, X. Jiang, J. Tang, J. Wang, G. Li, and Y. Jiang. 2008. Prevalence of plasmid-mediated AmpC beta-lactamases in a Chinese university hospital from 2003 to 2005: first report of CMY-2-type AmpC beta-lactamase resistance in China. J. Clin. Microbiol. 46:1317-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moland, E. S., N. D. Hanson, J. A. Black, A. Hossain, W. Song, and K. S. Thomson. 2006. Prevalence of newer beta-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J. Clin. Microbiol. 44:3318-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulvey, M. R., E. Bryce, D. A. Boyd, M. Ofner-Agostini, A. M. Land, A. E. Simor, and S. Paton. 2005. Molecular characterization of cefoxitin-resistant Escherichia coli from Canadian hospitals. Antimicrob. Agents Chemother. 49:358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadjar, D., M. Rouveau, C. Verdet, L. Donay, J. Herrmann, P. H. Lagrange, A. Philippon, and G. Arlet. 2000. Outbreak of Klebsiella pneumoniae producing transferable AmpC-type beta-lactamase (ACC-1) originating from Hafnia alvei. FEMS Microbiol. Lett. 187:35-40. [DOI] [PubMed] [Google Scholar]
- 14.Nasim, K., S. Elsayed, J. D. Pitout, J. Conly, D. L. Church, and D. B. Gregson. 2004. New method for laboratory detection of AmpC beta-lactamases in Escherichia coli and Klebsiella pneumoniae. J. Clin. Microbiol. 42:4799-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netzel, T. C., I. Jindani, N. Hanson, B. M. Turner, L. Smith, and K. H. Rand. 2007. The AmpC inhibitor, Syn2190, can be used to reveal extended-spectrum beta-lactamases in Escherichia coli. Diagn. Microbiol. Infect. Dis. 58:345-348. [DOI] [PubMed] [Google Scholar]
- 16.Pai, H., C. I. Kang, J. H. Byeon, K. D. Lee, W. B. Park, H. B. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2004. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-beta-lactamase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:3720-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Perez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitout, J. D., D. B. Gregson, D. L. Church, and K. B. Laupland. 2007. Population-based laboratory surveillance for AmpC beta-lactamase-producing Escherichia coli, Calgary. Emerg. Infect. Dis. 13:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin, X., S. J. Weissman, M. F. Chesnut, B. Zhang, and L. Shen. 2004. Kirby-Bauer disc approximation to detect inducible third-generation cephalosporin resistance in Enterobacteriaceae. Ann. Clin. Microbiol. Antimicrob. 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruppe, E., P. Bidet, C. Verdet, G. Arlet, and E. Bingen. 2006. First detection of the Ambler class C1 AmpC beta-lactamase in Citrobacter freundii by a new, simple double-disk synergy test. J. Clin. Microbiol. 44:4204-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahid, M., A. Malik, M. Agrawal, and S. Singhal. 2004. Phenotypic detection of extended-spectrum and AmpC beta-lactamases by a new spot-inoculation method and modified three-dimensional extract test: comparison with the conventional three-dimensional extract test. J. Antimicrob. Chemother. 54:684-687. [DOI] [PubMed] [Google Scholar]
- 22.Tan, T. Y., S. Y. Ng, L. Teo, Y. Koh, and C. H. Teok. 2008. Detection of plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis. J. Clin. Pathol. 61:642-644. [DOI] [PubMed] [Google Scholar]
- 23.Tracz, D. M., D. A. Boyd, R. Hizon, E. Bryce, A. McGeer, M. Ofner-Agostini, A. E. Simor, S. Paton, and M. R. Mulvey. 2007. ampC gene expression in promoter mutants of cefoxitin-resistant Escherichia coli clinical isolates. FEMS Microbiol. Lett. 270:265-271. [DOI] [PubMed] [Google Scholar]
- 24.Woodford, N., S. Reddy, E. J. Fagan, R. L. Hill, K. L. Hopkins, M. E. Kaufmann, J. Kistler, M. F. Palepou, R. Pike, M. E. Ward, J. Cheesbrough, and D. M. Livermore. 2007. Wide geographic spread of diverse acquired AmpC beta-lactamases among Escherichia coli and Klebsiella spp. in the UK and Ireland. J. Antimicrob. Chemother. 59:102-105. [DOI] [PubMed] [Google Scholar]