Abstract
Carbapenemases are among the newest resistance mechanisms to emerge in some gram-negative bacteria. We describe bacteremia in a critically ill liver transplant recipient infected with KPC-2-producing Enterobacter cloacae and Pseudomonas putida. Although this enzyme has been previously described in Enterobacter spp., this is the first report of KPC carbapenemase in P. putida.
Carbapenems are the broadest-spectrum β-lactam antibiotics and retain activity against many antibiotic-resistant organisms to include gram-negative organisms that produce extended-spectrum or AmpC β-lactamases (11). However, reports of carbapenem-hydrolyzing enzymes have become increasingly frequent in some locations in recent years (5). In the United States, the most common carbapenemases to emerge have been the Klebsiella pneumoniae carbapenemases (KPCs) (5). Since the first report of this plasmid-mediated carbapenemase in North Carolina, several outbreaks caused by KPC-producing isolates have been documented elsewhere, in particular in the northeastern United States (5, 11). KPC-producing isolates have also emerged in states outside of the northeastern United States to include Arkansas, Michigan, Missouri, Ohio, and Pennsylvania (12, 13). Clinical microbiology laboratories are becoming increasingly aware of the emergence of carbapenemase-producing organisms, but identification of such isolates remains difficult (2). In 2008, the Clinical and Laboratory Standards Institute (CLSI) suggested that KPC-producing isolates may display elevated carbapenem MICs of 2 or 4 μg/ml (6). Although these isolates are still considered “susceptible” based upon current CLSI interpretive criteria, they may not respond to carbapenem therapy (6). At present, the CLSI has not recommended a phenotypic test to confirm KPC production. We describe here simultaneous infection with KPC-2-producing Enterobacter cloacae and Pseudomonas putida in a critically ill liver transplant recipient.
A 54-year-old female was admitted to University Hospital to undergo an orthotopic liver transplant from an unrelated donor. Immediately after surgery, she required emergent surgical exploration due to hemorrhage. She experienced a complicated hospital course, including acute renal failure requiring hemodialysis, pulmonary embolus, and right lobe liver infarct. She had an open postsurgical abdominal wound following biliary anastamosis, right hepatic lobectomy, and repair of jejuno-jejunostomy. Due to a persistently elevated white blood cell count and the frequent need for vasopressor support, she received prolonged courses of broad-spectrum antibiotics to include 5 weeks of empirical meropenem and 7 weeks of linezolid. She also received trimethoprim-sulfamethoxazole, valgancyclovir, and antifungal prophylaxis throughout her hospital course in accordance with the local transplant protocol.
On hospital day 45, multidrug-resistant E. cloacae and P. putida with similar antibiotic susceptibility profiles grew from multiple blood cultures (Table 1) . Both isolates were susceptible to amikacin, and the patient was treated with that agent. Ciprofloxacin was added to the antibiotic regimen to broaden coverage; the E. cloacae demonstrated only intermediate susceptibility to this antimicrobial agent. The colistin MIC for the P. putida was 2 μg/ml, but for E. cloacae the MIC was >16 μg/ml based upon CLSI reference broth microdilution testing (7). The CLSI does not have specific colistin breakpoints for the Enterobacteriaceae or for P. putida, but a colistin MIC of ≤2 μg/ml is considered susceptible for P. aeruginosa and Acinetobacter spp., and a colistin MIC of >16 μg/ml would be considered resistant for both organisms (6). Subsequent to obtaining these blood isolates, both of these organisms grew from tissue cultures obtained during debridement of sacral and abdominal wounds. Proteus mirabilis, Stenotrophomonas maltophilia, and Escherichia coli were also isolated from the same wound cultures. Despite aggressive broad-spectrum antimicrobial therapy, the patient died 12 days after her first episode of bacteremia.
TABLE 1.
Antimicrobial agent susceptibilities of two clinical isolatesa
| Antimicrobial agent | Antimicrobial MIC (μg/ml) for:
|
|
|---|---|---|
| E. cloacae | P. putida | |
| Cefepime | >32 | >32 |
| Cefoxitin | >32 | >32 |
| Ceftazidime | >32 | >32 |
| Ceftriaxone | >32 | >32 |
| Piperacillin-tazobactam | >128 | >128 |
| Ciprofloxacin | 2 | >8 |
| Levofloxacin | 2 | >16 |
| Amikacin | 16 | 1 |
| Gentamicin | >8 | >8 |
| Tobramycin | >8 | >8 |
| Colistin | >16 | 2 |
| Ertapenem | >8 | >8 |
| Imipenem | >4 | >4 |
| Meropenem | >4 | >4 |
bla enzymes detected: E. cloacae, KPC-2 and SHV-12; and P. putida, KPC-2.
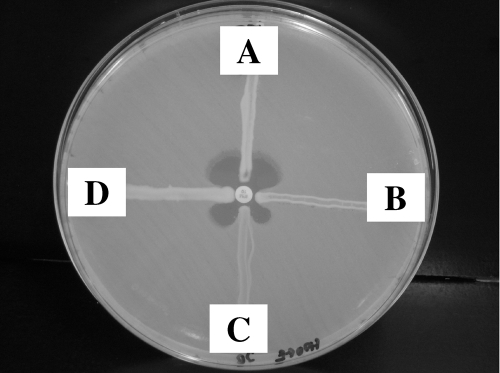
The E. cloacae and P. putida isolates were both initially identified using a Vitek 2 instrument (bioMérieux, Hazelwood, MO). The identifications were confirmed by performing 16S rRNA sequencing (15). Because of resistance to meropenem and to all other β-lactams tested, both isolates were screened for the presence of a carbapenemase using the modified Hodge test (2). Both strains demonstrated carbapenem hydrolysis using imipenem as the test substrate (Fig. 1). PCR amplification of DNA extracts using previously described primers and test conditions for various extended-spectrum β-lactamases and KPCs were performed, followed by sequencing of the PCR products (9, 12). This revealed the presence of blaKPC-2 in both isolates and blaSHV-12 in the E. cloacae.
FIG. 1.
Modified Hodge test using a 10-μg imipenem disk. Isolate A (K. pneumoniae, ATCC 700603) does not produce a carbapenemase and is negative by this test. Isolates B (P. putida), C (E. cloacae), and D (K. pneumoniae, CAP D05-07) all produce KPC-2 and are positive by this test.
Our patient suffered from bacteremia due to KPC-2-producing E. cloacae and P. putida recovered simultaneously from multiple cultures, and she eventually died. Although the initial source of infection was undetermined, both organisms were isolated from wound cultures as well as from blood. It is possible that transfer of the plasmid encoding the carbapenemase could have occurred in the milieu of the mixed wound infection. Prior to this patient's infection, no KPC-producing isolates had been recovered in this hospital, and no others have been detected since this case.
In the United States, KPC enzymes have emerged as a major clinical concern among some members of the Enterobacteriaceae, but these enzymes have rarely been described outside of that family (5, 11). Recently, three Pseudomonas aeruginosa clinical isolates from Colombia were found to express KPC-2; however, we believe that the present study represents the first report of KPC production in P. putida (14). Infections caused by P. putida are relatively rare and are generally restricted to immunocompromised patients and patients with invasive medical devices in place (4). Although not previously recognized to produce KPC, this member of the fluorescent group of pseudomonads is often resistant to fluoroquinolones, aminoglycosides, and various β-lactams (1, 3, 8, 10). Previously described carbapenem-resistant P. putida isolates have been associated with production of IMP- or VIM-type metallo-β-lactamases, but not KPCs (1, 3, 8, 10).
Nonsusceptibility to colistin has been described among the Enterobacteriaceae. (5). When present in combination with a carbapenemase, therapeutic options are extremely limited. It is particularly unusual that this isolate was nonsusceptible given that this patient had not been exposed to colistin during her hospital course. Susceptibility to colistin should not be assumed, and appropriate testing should be performed when therapy with this potentially toxic antimicrobial agent is contemplated.
These appear to represent the first KPC-producing clinical isolates in Texas, as well as the first occurrence of blaKPC-2 in P. putida. This extends the host range for the KPC-2 β-lactamase into another Pseudomonas species. Microbiologists and clinicians should be aware that carbapenemases can appear in several different species and in different gram-negative bacterial families. Practical phenotypic screening and confirmatory tests are needed to facilitate the timely detection of such strains by clinical microbiology laboratories.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the U.S. government.
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Almuzara, M., M. Radice, N. de Gárate, A. Kossman, A. Cuirolo, G. Santella, A. Famiglietti, G. Gutkind, and V. Vay. 2007. VIM-2-producing Pseudomonas putida, Buenos Aires. Emerg. Infect. Dis. 13:668-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, K. F., D. R. Lonsway, J. K. Rasheed, J. Biddle, B. Jensen, L. K. McDougal, R. B. Carey, A. Thompson, S. Stocker, B. Limbago, and J. B. Patel. 2007. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J. Clin. Microbiol. 45:2723-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boagaerts, P., T. Huang, H. Rodriguez-Villalobos, C. Bauraing, A. Deplano, M. J. Struelens, and Y. Glupczynski. 2008. Nosocomial infections caused by multidrug-resistant Pseudomonas putida isolates producing VIM-2 and VIM-4 metallo-β-lactamases. J. Antimicrob. Chemother. 61:749-751. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter, R. J., J. D. Hartzell, J. A. Forsberg, B. S. Babel, and A. Ganesan. 2008. Pseudomonas putida war wound infection in a US marine: a case report and review of the literature. J. Infect. 56:234-240. [DOI] [PubMed] [Google Scholar]
- 5.Castanheira, M., H. S. Sader, L. M. Deshpande, T. R. Fritsche, and R. N. Jones. 2008. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-β-lactamase-producing Enterobacteriaceae: report form the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 52:570-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. Approved standard M100-S18. CLSI, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. CLSI, Wayne, PA.
- 8.Horii, T., H. Muramatsu, and Y. Iinuma. 2005. Mechanisms of resistance to fluoroquinolones and carbapenems in Pseudomonas putida. J. Antimicrob. Chemother. 56:643-647. [DOI] [PubMed] [Google Scholar]
- 9.Lewis, J. S., II, M. Herrera, B. Wickes, J. E. Patterson, and J. H. Jorgensen. 2007. First report of the emergence of CTX-M-type extended spectrum β-lactamases (ESBLs) as the predominant ESBL isolated in a U.S. health care system. Antimicrob. Agents Chemother. 51:4015-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendes, R. E., M. Castanheira, M. A. Toleman, H. S. Sader, R. N. Jones, and T. R. Walsh. 2007. Characterization of an integron carrying blaimp-1 and a new aminoglycoside resistance gene, aac(6′)-31, and its dissemination among genetically unrelated clinical isolates in a Brazilian hospital. Antimicrob. Agents Chemother. 51:2611-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasheed, J. K., J. W. Biddle, K. F. Anderson, L. Waisher, C. Chenoweth, J. Perrin, D. W. Newton, and J. B. Patel. 2008. Detection of the Klebsiella pneumoniae carbapenemase type 2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J. Clin. Microbiol. 46:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibbetts, R., J. G. Frye, J. Marschall, D. Warren, and W. Dunne. 2008. Detection of KPC-2 in a clinical isolate of Proteus mirabilis and first reported description of carbapenemase resistance caused by a KPC β-lactamase in P. mirabilis. J. Clin. Microbiol. 46:3080-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villegas, M. V., K. Lolans, A. Correa, J. N. Kattan, J. A. Lopez, J. P. Quinn, et al. 2007. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob. Agents Chemother. 51:1553-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe, K., Y. Kodama, and S. Harayama. 2001. Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J. Microbiol. Methods 44:253-262. [DOI] [PubMed] [Google Scholar]