Abstract
We demonstrate that xylitol can be added to polymethylmethacrylate (PMMA) bone cement to enhance the elution of daptomycin in terms of both the peak and sustained release of antibiotic. We also demonstrate that a PMMA-xylitol formulation optimized for daptomycin can be used to enhance the elution of both vancomycin and gentamicin.
The treatment of chronic musculoskeletal and implant-associated infection requires a multifactorial approach that can include systemic antibiotic therapy, the surgical debridement of the affected site, including the removal of infected devices, and local antibiotic therapy targeted directly to the site of infection (1). The latter often is done by incorporating antibiotics into handmade polymethylmethacrylate (PMMA) beads (8). Elution profiles from antibiotic-laden PMMA beads can be modified by the incorporation of biologically inert fillers that increase both the rate and duration of antibiotic release (2-5). One such filler is glycine; indeed, PMMA beads containing glycine and gentamicin are commercially available in Europe under the trade name Septopal (Biomet Europe, Dordrecht, The Netherlands), and these beads exhibit an enhanced elution profile compared to that of handmade beads lacking glycine (6). However, in previous studies we found that xylitol enhances elution to a greater extent than glycine (3). The experiments described in this report were aimed at optimizing the use of xylitol as a means of enhancing the elution of antibiotics from PMMA.
To evaluate the impact of xylitol on the elution of daptomycin from PMMA, a 40-g packet of Palacos-R PMMA (Zimmer, Inc., Dover, OH) powder was hand mixed with the designated amounts of daptomycin with or without xylitol crystals (Sigma Chemical Co., St. Louis, MO). Polymerization was done using a single vial of monomer irrespective of the presence or amount of xylitol. Each PMMA preparation was mixed until the cement was evenly thickened and then formed into beads using a 7-mm bead template (Wright Medical Technology, Arlington, TN). Beads prepared using each formulation were divided into sample sets of four beads per set. Each set was placed in a sterile 25-ml Erlenmeyer flask containing 6 ml of sterile phosphate-buffered saline (PBS). Each flask then was incubated at 37°C with constant shaking for 10 days. At 24-h intervals, the entire volume of PBS was removed for analysis and replaced with 6 ml of fresh, sterile PBS. Samples were stored at −20°C until the entire elution series for each PMMA formulation had been collected.
The amount of daptomycin in each sample was determined using a bioassay. Briefly, a fresh preparation of daptomycin was prepared in sterile PBS on each analysis day. This stock solution was used to prepare twofold dilutions in a 96-well microtiter plate. A twofold dilution series also was prepared with each experimental sample. Dilutions were prepared in tryptic soy broth (TSB) supplemented with 2.5 mM CaCl2. Each well was inoculated subsequently with 104 CFU of a daptomycin-sensitive strain of Staphylococcus aureus (UAMS-1; daptomycin MIC, ≤0.5 μg/ml). After incubation at 37°C for 24 h, the concentration of active antibiotic in each experimental sample was assessed by comparison to the results obtained in the same assay with the daptomycin standard. Results obtained with the bioassay were confirmed by measuring the total amount of daptomycin in each sample by high-pressure liquid chromatography (HPLC) using a C8 column and an acidic acetonitrile-salt mobile phase (7).
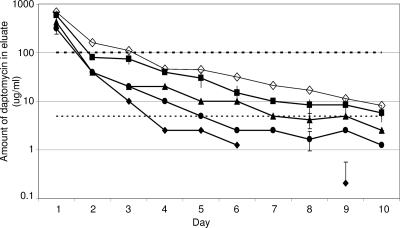
We chose as our goal an elution profile that included a peak concentration at least 100× and a sustained concentration (defined at 10 days postelution) at least 5× the MIC defined by the CLSI as the breakpoint for a daptomycin-sensitive strain of S. aureus (≤1.0 μg/ml). Based on our earlier experiments demonstrating that the incorporation of 1.0 g of daptomycin and 28.0 g of xylitol failed to yield an elution profile that met either of these objectives (3), we initially used 2.0 g of daptomycin and 0, 7, 14, or 28 g of xylitol per 40-g packet of PMMA. Based on two-way analysis of variance with Tukey post hoc adjustments (SigmaStat, version 2; SPSS Inc., Chicago, IL), we confirmed a significant difference (P ≤ 0.001) not only between PMMA and PMMA containing as little as 7 g of xylitol but also between PMMA formulations containing increasing amounts of xylitol. While all formulations, including the one that lacked xylitol, yielded a peak concentration of ≥100 μg/ml, the only formulation that yielded a sustained concentration at least 5× the MIC was the one containing 28 g of xylitol (Fig. 1).
FIG. 1.
Elution from PMMA beads containing 2.0 g daptomycin as a function of xylitol. Assays were done with PMMA beads containing 2 g of daptomycin and 0 (⧫), 7 (•), 14 (▴), or 28 (▪) g of xylitol per 40-g packet of PMMA. The amount of daptomycin present in the eluate was determined by bioassay. Results are reported as the averages ± the standard deviations from three experiments. Missing data points in the series without xylitol (⧫) are indicative of daptomycin levels below the level of detection. Results obtained by HPLC with the formulation containing 28 g of xylitol are shown for comparison (⋄). The upper dashed line indicates the 100× threshold (100 μg/ml), while the lower dashed line indicates the 5× threshold (5 μg/ml). Statistical analysis confirmed a significant difference (P ≤ 0.05) between PMMA with (•) and without (⧫) 7 g of xylitol and between PMMA formulations containing increasing amounts of xylitol (•, ▴, and ▪).
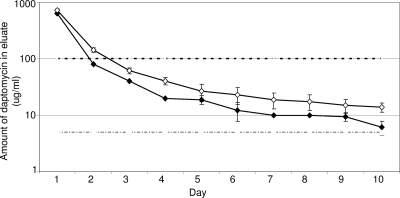
We next carried out an experiment using formulations containing 2.0 g of daptomycin and 18 or 22 g of xylitol per 40-g packet of PMMA. The results demonstrated that the 100× elution objective was met with either formulation, but that 18 g of xylitol was not sufficient with respect to the 5× objective (data not shown). In contrast, a PMMA formulation containing 2 g of daptomycin and 22 g of xylitol yielded a peak concentration of ≥640 μg/ml and a sustained concentration of ≥6.11 μg/ml (Fig. 2). These results were confirmed by HPLC analysis (Fig. 2).
FIG. 2.
Elution profile with the optimized formulation of PMMA-xylitol for the elution of daptomycin. Samples from PMMA beads containing 2 g of daptomycin and 22 g of xylitol per 40-g packet of PMMA were assayed by HPLC (⋄) or bioassay (⧫). Results are reported as the averages ± standard deviations from six replicates. The upper dashed line indicates the 100× threshold (100 μg/ml), while the lower dashed line indicates the 5× threshold (5 μg/ml).
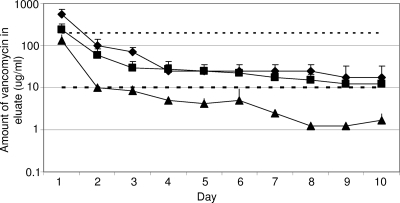
Based on these results, we concluded that a PMMA formulation containing 2 g of daptomycin and 22 g of xylitol per 40-g packet of PMMA can be considered optimal, at least as defined by in vitro elution experiments. We therefore evaluated whether a PMMA formulation containing 22 g of xylitol could be used to enhance the elution of vancomycin and gentamicin. Zalavras et al. (8) recommended the use of 4.0 g of vancomycin or 3.6 g of gentamicin per 40-g packet of PMMA, and we based our experiments on these recommendations. With 4.0 g of vancomycin, xylitol was not necessary to achieve either of our elution objectives (Fig. 3). However, subsequent experiments demonstrated that the inclusion of xylitol enhanced elution to the point that both also could be met with only 2.0 g of vancomycin. Specifically, a PMMA formulation containing 2 g of vancomycin and 22 g of xylitol yielded a peak concentration on day 1 of ≥240 μg/ml and a sustained concentration on day 10 of ≥12.5 μg/ml (Fig. 3). These values represent 120× and 6.25× the vancomycin MICs defined for a sensitive strain (≤2 μg/ml).
FIG. 3.
Elution of vancomycin from PMMA as a function of xylitol. Comparison of PMMA beads containing 4 g of vancomycin (⧫) with PMMA beads containing 2 g of vancomycin with (▪) and without (▴) 22 g of xylitol per 40-g packet of PMMA. Results are reported as the averages ± standard deviations from three assays. The upper dashed line indicates the 100× threshold (200 μg/ml), while the lower dashed line represents the 5× threshold (10 μg/ml). Statistical analysis confirmed a significant difference (P ≤ 0.05) between PMMA containing 2 g of vancomycin with (▪) and without (▴) xylitol. In contrast, there was no significant difference between a PMMA formulation containing 4 g of vancomycin without xylitol (⧫) and 2 g of vancomycin with xylitol (▪).
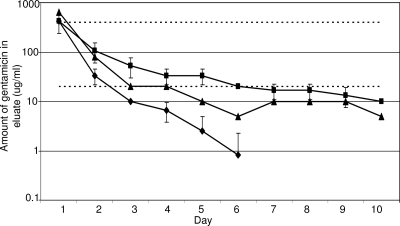
Compared to results for the use of daptomycin and vancomycin, the inclusion of xylitol had relatively little impact on the elution of gentamicin from PMMA. Specifically, the peak concentration of ≥426.7 μg/ml exceeded the 100× MIC objective (400 μg/ml) and was unchanged in the presence of xylitol. However, the sustained concentrations failed to achieve the 5× objective (20 μg/ml) irrespective of the inclusion of xylitol (Fig. 4). Nevertheless, the addition of xylitol did enhance elution, as evidenced by the fact that a PMMA formulation containing 3.6 g of gentamicin but without xylitol fell below the 5× objective by day 3 and reached undetectable levels by day 7 (Fig. 4). In contrast, a formulation containing 3.6 g of gentamicin and 22 g of xylitol yielded an elution profile that met or exceeded the 5× threshold through day 6 and yielded detectable levels of antibiotic (10 μg/ml) even on day 10 (Fig. 4). Moreover, this was true even when the amount of gentamicin was reduced from 3.6 to 2.0 g per 40 g of PMMA. In fact, PMMA formulations containing xylitol were the only ones in which the amount of antibiotic observed on day 10 remained above the CSLI breakpoint MIC (≤4.0 μg/ml) for a gentamicin-sensitive strain of S. aureus. Subsequent analysis confirmed that the differences observed in the elution profile of PMMA with and without xylitol were statistically significant (P ≤ 0.001).
FIG. 4.
Elution of gentamicin from PMMA as a function of xylitol. Assays were done with PMMA beads containing 3.6 g of gentamicin with (▪) or without (⧫) 22 g of xylitol per 40-g packet of PMMA. Results obtained with 2.0 g of gentamicin and 22 g of xylitol are shown for comparison (▴). Results are reported as the averages ± standard deviations from three assays. The upper dashed line indicates the 100× threshold (400 μg/ml), while the lower dashed line represents the 5× threshold (20 μg/ml). Statistical analysis confirmed a significant difference (P ≤ 0.05) between PMMA formulations containing 3.6 g of gentamicin with (▪) and without (⧫) xylitol and between a PMMA formulation containing 3.6 g of gentamicin without xylitol (⧫) and a PMMA formulation containing 2 g of gentamicin with xylitol (▴).
Taken together, the results we report demonstrate that the addition of 22 g of xylitol to a 40-g packet of PMMA enhances the elution profiles of daptomycin and, to a somewhat lesser extent, vancomycin and gentamicin. This was true in terms of both peak and sustained concentrations of antibiotic. The inclusion of xylitol did not necessitate the alteration of the standard method for preparing PMMA beads, in that the single vial of monomer provided by the manufacturer remained sufficient to achieve polymerization. The verification of the significance of these in vitro observations will require in vivo studies taking into account both the complete resolution of the infection and the time required to achieve this outcome, but taken together we believe the results we report support the hypothesis that PMMA beads containing xylitol offer a therapeutic advantage over the current clinical practice.
Acknowledgments
This research was supported by funding from Cubist Pharmaceuticals (M.S.S.) and the AI069087 from the National Institute of Allergy and Infectious Disease (M.S.S.).
We thank Sonja Daily for expert technical assistance.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Lew, D. P., and F. A. Waldvogel. 2004. Osteomyelitis. Lancet 364:369-379. [DOI] [PubMed] [Google Scholar]
- 2.McLaren, A. C., S. G. McLaren, and M. K. Hickmon. 2007. Sucrose, xylitol and erythritol increase PMMA permeability for depot antibiotics. Clin. Orthop. Relat. Res. 461:60-63. [DOI] [PubMed] [Google Scholar]
- 3.McLaren, A. C., S. G. McLaren, and M. S. Smeltzer. 2006. Xylitol and glycine fillers increase permeability of PMMA to enhance elution of daptomycin. Clin. Orthop. Relat. Res. 451:25-28. [DOI] [PubMed] [Google Scholar]
- 4.McLaren, A. C., C. L. Nelson, S. G. McLaren, and G. R. DeCLerk. 2004. The effect of glycine filler on the elution rate of gentamicin from acrylic bone cement: a pilot study. Clin. Orthop. Relat. Res. 427:25-27. [DOI] [PubMed] [Google Scholar]
- 5.Nelson, C. L. 2004. The current status of material used for depot delivery of drugs. Clin. Orthop. Relat. Res. 427:72-78. [DOI] [PubMed] [Google Scholar]
- 6.Nelson, C. L., F. M. Griffin, B. H. Harrison, and R. E. Cooper. 1992. In vitro elution characteristics of commercially and noncommercially prepared antibiotic PMMA beads. Clin. Orthop. Relat. Res. 284:303-309. [PubMed] [Google Scholar]
- 7.Richelsoph, K. C., N. D. Webb, and W. O. Haggard. 2007. Elution behavior of daptomycin-loaded calcium sulfate pellets: a preliminary study. Clin. Orthop. Relat. Res. 461:68-73. [DOI] [PubMed] [Google Scholar]
- 8.Zalavras, C. G., M. J. Patzakis, and P. Holtom. 2004. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin. Orthop. Relat. Res. 427:86-93. [DOI] [PubMed] [Google Scholar]