Abstract
Two neuraminidase (NA) inhibitors, zanamivir (Relenza) and oseltamivir phosphate (Tamiflu), have been licensed for the treatment of and prophylaxis against influenza. In this paper, the new potent NA inhibitor R-125489 is reported for the first time. R-125489 inhibited the NA activities of various type A and B influenza viruses, including subtypes N1 to N9 and oseltamivir-resistant viruses. The survival effect of R-125489 was shown to be similar to that of zanamivir when administered intranasally in a mouse influenza virus A/Puerto Rico/8/34 infection model. Moreover, we found that the esterified form of R-125489 showed improved efficacy compared to R-125489 and zanamivir, depending on the acyl chain length, and that 3-(O)-octanoyl R-125489 (CS-8958) was the best compound in terms of its life-prolonging effect (P < 0.0001, compared to zanamivir) in the same infection model. A prolonged survival effect was observed after a single administration of CS-8958, even if it was given 7 days before infection. It is suggested that intranasally administered CS-8958 works as a long-acting NA inhibitor and shows in vivo efficacy as a result of a single intranasal administration.
Influenza is a serious respiratory illness which can be debilitating and can cause complications that lead to hospitalization and death, especially in the elderly. This respiratory disease is caused by influenza A and B viruses that are highly contagious pathogens for humans. Influenza A viruses are classified into subtypes based on the antigenicity of hemagglutinin (HA) and neuraminidase (NA) molecules. To date, 16 HA subtypes (H1 to H16) and 9 NA subtypes (N1 to N9) have been described, but only a limited number of subtypes (H1N1, H2N2, and H3N2) have circulated in humans (18). Currently, seasonal influenza or influenza epidemics are caused by influenza A virus subtypes H1N1 and H3N2 and influenza B virus (18). Every year, the global burden of influenza epidemics is believed to be 3.5 million cases of severe illness and 300,000 to 500,000 deaths (4).
In the last 100 years, humans have experienced three influenza pandemics, the first in 1918 (H1N1), the second in 1957 (H2N2), and the third in 1968 (H3N2) (18). Since 2003, the number of human infections from the highly pathogenic H5N1 virus has increased, along with the fatality rate. More than 385 cases of infection with the H5N1 virus and as many as 243 deaths have been reported as of 19 June 2008 (World Health Organization, confirmed human cases of avian influenza A(H5N1) [http://www.who.int/csr/disease/avian_influenza/country/en/index.html]). Human infections from new viruses with other HA- and NA-subtype combinations have been reported as well (World Health Organization, avian influenza (“bird flu”): fact sheet [http://www.who.int/csr/disease/avian_influenza/avianinfluenza_factsheetJan2006/en/index.html#humans]). Animal influenza viruses with subtypes different from those of human viruses are thought to be reservoirs for new viruses that may affect humans.
Presently, there are two classes of anti-influenza drugs available for the treatment of influenza, the M2 ion channel inhibitors and the NA inhibitors. M2 ion channel inhibition is involved either in initiating the infection or in assembling influenza A, but not B, virus replication (7). However, this class of drugs has been shown to rapidly generate viral resistance in treated patients (2, 3) and has not been recommended for use in the treatment of or chemoprophylaxis against influenza in the United States since the 2005 influenza season (2, 3). The second and most recently developed class of influenza A and B antiviral drugs is the NA inhibitor, which bind to the NA surface glycoprotein of newly formed virus particles and prevent their efficient release from the host cell (5). Currently, two NA inhibitors, zanamivir (Relenza) (inhaled drug; 10 mg/dose) and oseltamivir phosphate (Tamiflu) (oral drug; 75 mg/dose) are licensed. Both drugs require twice-daily administrations for treatment. Oseltamivir is the predominant choice and is used worldwide for the treatment of influenza, but the generation and circulation of oseltamivir-resistant mutants of seasonal influenza, as well as H5N1 avian influenza, have become major concerns (6, 8, 11, 12, 13).
We discovered the new, potent NA inhibitor R-125489, which has NA inhibitory activities against various influenza A and B viruses, including the subtypes N1 to N9 and oseltamivir-resistant viruses, and shows a prolonged survival effect similar to that of zanamivir in a mouse influenza virus A/Puerto Rico/8/34 (A/PR/8/34) lethal infection model. Moreover, we found that a single administration of its esterified forms dramatically improved the prolonged-survival effect in the same model and that 3-(O)-octanoyl R-125489 (CS-8958) is the best compound in terms of its life-prolonging effect.
MATERIALS AND METHODS
Test compounds.
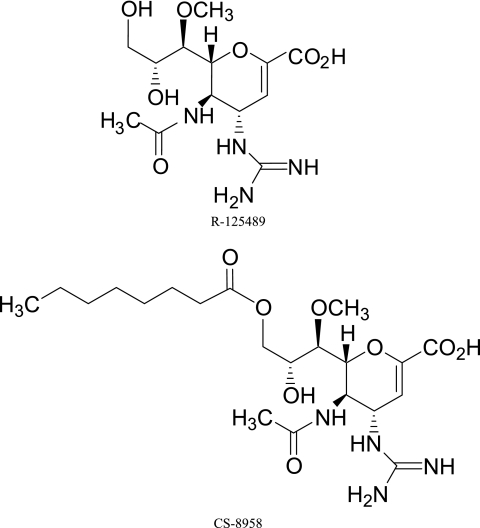
Compound R-125489 and the prodrugs of R-125489 were used with acyl chains of various lengths (butyl, R-125489-C4; hexanoyl, R-125489-C6; octanoyl, R-125489-C8 [CS-8958]; decanoyl, R-125489-C10; tetradecanoyl, R-125489-C14; and hexadecanoyl, R-125489-C16) at position 3 were synthesized utilizing previously published procedures (9, 10) and provided by Daiichi Sankyo Co., Ltd. Zanamivir (molecular weight [MW], 332.31) was synthesized, and oseltamivir carboxylate (MW, 284.35) was prepared from oseltamivir phosphate extracted from the commercial preparation Tamiflu by the same company. The chemical structures of R-125489 (MW, 346.34) and CS-8958 {(4S,5R,6R)-5-acetamido-4-guanidino-6-[(1R,2R)-2-hydroxy-1-methoxy-3-(octanoyloxy)propyl]-5,6-dihydro-4H-pyran-2-carboxylic acid; MW, 472.53} are shown in Fig. 1.
FIG. 1.
Chemical structures of R-125489 and CS-8958.
Cells.
Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC CCL-34). HeLa cells (ATCC CCL-2) and MOLT-4 cells (ATCC CRL-1582) were purchased from DS Pharma Biomedical Co., Ltd. (Japan). MDCK and HeLa cells were maintained in minimum essential medium containing 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. MOLT-4 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. The cells were cultured in 5% CO2 at 37°C.
Viruses.
The laboratory strains and vaccine strains were provided by the National Institute of Infectious Diseases, Japan. The clinical isolates of human influenza viruses were from various prefectural institutes in Japan (see Acknowledgments). The animal influenza viruses were from the Graduate School of Veterinary Medicine, Hokkaido University, Japan. The H274Y mutant was from the Yokohama City Institute of Health. The other mutants were from the Division of Virology, Department of Microbiology and Immunology, the Institute of Medical Science, University of Tokyo. The viruses were grown once in fertilized eggs or MDCK cells.
Other reagents.
The NAs of Vibrio cholerae (type II) and Clostridium perfringens (type X) were purchased from Sigma Chemical Co. (St. Louis, MO), and the NA of Newcastle disease virus was from Calbiochem-Novabiochem Corporation (San Diego, CA). The ammonium salt 4-methylumbelliferyl-N-acetyl-α-d-neuraminic acid (4-MU-NANA) was purchased from Nacalai Tesque, Inc., Japan, and used as the substrate for the NA.
Inhibition of influenza virus NA.
The NA activity was measured according to the method described by Bantia et al. (1). The virus solution and the test compound were dissolved in distilled water at various concentrations and mixed at a final concentration of 32.5 mM 2-(N-morpholino)ethanesulfonic acid-NaOH buffer (pH 6.5) containing 4 mM CaCl2 at a volume of 90 μl, and the mixture was preincubated at room temperature for 10 min. Then, 10 μl of 1 mM 4-MU-NANA (final concentration of 100 μM) was added to start the reaction, and the mixture was incubated for another 30 min at room temperature. After the reaction, the fluorescence was measured with the excitation at 360 nm and the emission at 460 nm, using a CytoFluor series 4000 instrument (Applied Biosystems Japan, Ltd.). Under these experimental conditions, a linear relationship between the reaction time, enzyme amount, and fluorescence was confirmed, and the volume of the virus solution was used to give 10 to 100 pmol/min of 4-methylumbelliferone, an enzyme reaction product. The NA inhibition assay was done in triplicate.
The percentage of residual enzyme activity was calculated using the fluorescence values for the background (without virus) and the fluorescence value for the control (without compounds). For each virus and compound, the contiguous concentrations (i.e., 1.0, 3.0, and 10 nM) at which the mean of the residual enzyme activities were between 10% and 90% were selected. The 50% inhibitory concentration (IC50) was estimated by linear regression analysis using the residual enzyme activities (n = 3) and the logarithmically transformed concentrations selected as described above. These calculations were carried out using SAS System Release version 8.2 (SAS Institute Inc.) software.
Efficacy in a mouse influenza virus infection model.
The in vivo experiments were performed as described by Ryan et al. (17). Female BALB/c mice (5 to 6 weeks old, specific pathogen free; Japan SLC, Inc., or Charles River Laboratories Japan, Inc.) were anesthetized with ether-CHCl3 (1:1) and infected intranasally with 50 μl of A/PR/8/34 virus (500 or 1,500 PFU/mouse; 1 50% lethal dose = 27 PFU) diluted with phosphate-buffered saline containing 0.42% bovine serum albumin (day 0). The anesthetized mice were intranasally administered a 50-μl dose of the indicated test compounds dissolved in saline at the times indicated in the legends to Fig. 2 to 4. Each administration group contained 10 to 12 mice. The other experimental conditions specific to the experiment are described in the legends to Fig. 1 to 4. We observed the surviving mice daily from day 0 to day 20. The mice were kept in an environmentally controlled room throughout the experiments. The rearing conditions of the room were as follows: temperature, 23° ± 2°C; relative humidity, 55% ± 10%; and light/dark cycle, 12 h. All the experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd.
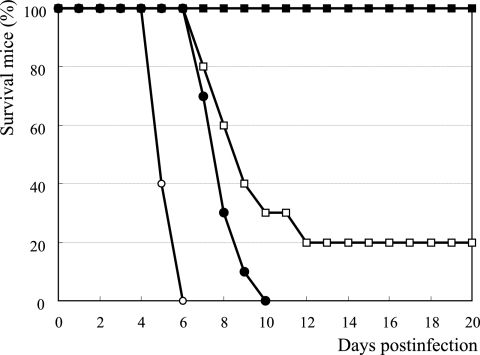
FIG. 2.
In vivo efficacy of CS-8958 and R-125489 in a mouse influenza virus infection model. Influenza A virus A/PR/8/34-infected (500 PFU, 0 h) mice were given 0.2 μmol/kg of CS-8958 (filled squares), R-125489 (open squares), zanamivir (filled circles), and saline (open circles) at 4 h before infection and 4 h and 18 h p.i. The dose of 0.2 μmol/kg corresponded to 95, 69, and 66 μg/kg for CS-8958, R-125489, and zanamivir, respectively. The number of surviving mice was monitored for 20 days p.i. (n = 10). (Results were not significant for the R-125489 administration group versus the zanamivir administration group [P = 0.0732; log rank test]).
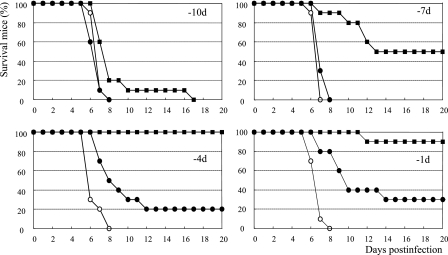
FIG. 4.
In vivo efficacy of the prophylactic intranasal administration of CS-8958 or zanamivir in a mouse influenza virus infection model. An intranasal dose of 0.5 μmol/kg of CS-8958 (filled squares), zanamivir (filled circles), or saline (open circles) was intranasally administered once at 10, 7, 4, or 1 day before infection (−10d, −7d, −4d, and −1d), and mice were infected with influenza A (A/PR/8/34) virus at 500 PFU/mouse on day 0. The dose of 0.5 μmol/kg corresponded to 236 μg/kg for CS-8958 and 166 μg/kg for zanamivir. The number of surviving mice was monitored for 20 days p.i. (n = 10). (Results were significant for the group given zanamivir at −1 day versus the group given saline [P = 0.0151]. Results were not significant for the three other zanamivir administration groups versus the saline administration group. Results were significant for all CS-8958 groups [P values of 0.0230 for the group dosed at −10 days and <0.0001 for the other three groups; log rank test]).
Statistical analysis.
The percentage of mice that survived and the median survival times were calculated by the Kaplan-Meier method using survival time data for each mouse. A log rank test based on the joint-ranking method was carried out to compare the life-prolonging effects among the groups. A statistical adjustment for multiple comparisons was done by the Bonferroni method. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS System Release version 8.2 software.
RESULTS
Inhibition of NA activity of human influenza viruses.
The IC50s of the NA inhibitors to the laboratory strains, vaccine strains, and clinical isolates of the influenza viruses are shown in Table 1. R-125489 showed potent NA inhibitory activity with IC50s of 1.29 to 5.97 nM (mean, 2.49 nM) to 11 strains of H1N1 viruses. Zanamivir and oseltamivir carboxylate had inhibitory activities with IC50s of 0.751 to 3.62 nM (mean, 1.64 nM) and 0.658 to 2.39 nM (mean, 1.34 nM), respectively. However, CS-8958 showed only weak inhibitory activities with IC50s of 631 to 1,170 nM (mean, 822 nM). R-125489 maintained potent activity against the 1934 isolate (A/PR/8/34) through the recent 2006 isolate.
TABLE 1.
IC50s and 95% CIs of NA activities against human influenza virusesc
| Influenza virusa | Virus subtype or lineage | Strain typeb | CS-8958
|
R-125489
|
Zanamivir
|
Oseltamivir carboxylate
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | 95% CI | IC50 (nM) | 95% CI | IC50 (nM) | 95% CI | IC50 (nM) | 95% CI | |||
| H1N1 viruses | ||||||||||
| A/Puerto Rico/8/1934 | H1N1 | LS | 931 | 829-1,050 | 5.97 | 4.88-7.69 | 3.62 | 3.08-4.30 | 2.39 | 1.96-2.95 |
| A/Yamagata/32/1989 | H1N1 | VS | 882 | 776-998 | 3.42 | 2.94-4.01 | 2.55 | 1.96-3.67 | 1.54 | 1.28-1.91 |
| A/New Caledonia/20/1999 | H1N1 | VS | 666 | 589-756 | 2.32 | 1.85-2.95 | 2.05 | 1.68-2.65 | 1.58 | 1.35-1.90 |
| A/Shiga/1/2002 | H1N1 | CI | 733 | 630-860 | 2.09 | 1.39-3.45 | 1.63 | 1.15-2.40 | 1.34 | 1.05-1.72 |
| A/Yamagata/3/2002 | H1N1 | CI | 709 | 592-858 | 2.57 | 1.93-3.55 | 1.68 | 1.36-2.06 | 1.08 | 0.944-1.23 |
| A/Yamagata/57/2002 | H1N1 | CI | 1,170 | 1,040-1,320 | 1.65 | 1.39-2.02 | 1.14 | 0.832-1.70 | 0.658 | 0.575-0.756 |
| A/Saitama/78/2003 | H1N1 | CI | 940 | 756-1,170 | 1.79 | 1.26-2.55 | 1.38 | 1.03-1.99 | 1.12 | 0.775-1.71 |
| A/Aichi/193/2004 | H1N1 | CI | 983 | 792-1,220 | 2.63 | 2.32-3.08 | 1.17 | 0.796-2.00 | 1.26 | 0.789-2.54 |
| A/Okinawa/42/2004 | H1N1 | CI | 631 | 533-750 | 2.07 | 1.33-4.09 | 0.751 | 0.527-1.13 | 1.86 | 1.69-2.05 |
| A/Aichi/169/2005 | H1N1 | CI | 664 | 545-817 | 1.55 | 1.06-2.38 | 1.19 | 0.798-2.06 | 1.12 | 0.780-1.81 |
| A/Yamagata/83/2006 | H1N1 | CI | 733 | 668-808 | 1.29 | 0.910-2.06 | 0.880 | 0.686-1.17 | 0.785 | 0.660-0.945 |
| Mean ± SD | 822 ± 171 | 2.49 ± 1.30 | 1.64 ± 0.838 | 1.34 ± 0.491 | ||||||
| H2N2 virus | ||||||||||
| A/Singapore/1/1957 | H2N2 | LS | 128 | 107-152 | 11.4 | 9.14-14.4 | 3.66 | 2.98-4.56 | 0.925 | 0.831-1.03 |
| H3N2 virus | ||||||||||
| A/Aichi/2/1968 | H3N2 | LS | 164 | 149-179 | 16.8 | 13.9-21.1 | 7.33 | 5.75-10.2 | 2.39 | 1.83-3.48 |
| A/Kitakyushu/159/1993 | H3N2 | VS | 221 | 205-239 | 23.5 | 18.9-31.3 | 9.30 | 8.40-10.3 | 1.60 | 1.14-2.60 |
| A/Wyoming/03/2003 | H3N2 | VS | 114 | 99.5-129 | 22.2 | 17.9-28.0 | 11.0 | 9.50-12.9 | 1.71 | 1.34-2.34 |
| A/Wellington/1/2004 | H3N2 | VS | 157 | 132-183 | 26.8 | 23.9-30.9 | 8.74 | 7.47-10.2 | 1.99 | 1.92-2.06 |
| A/California/07/2004 | H3N2 | VS | 135 | 121-150 | 19.4 | 15.7-25.6 | 8.21 | 6.32-11.8 | 1.90 | 1.47-2.69 |
| A/New York/55/2004 | H3N2 | VS | 136 | 125-147 | 19.2 | 15.3-25.9 | 7.35 | 5.68-10.5 | 1.56 | 1.18-2.28 |
| A/Hiroshima/52/2005 | H3N2 | VS | 69.1 | 62.5-76.7 | 12.7 | 11.1-14.6 | 5.11 | 4.32-6.24 | 1.33 | 1.18-1.51 |
| A/Wisconsin/67/2005 | H3N2 | VS | 187 | 171-206 | 38.8 | 33.4-45.5 | 14.2 | 11.9-17.2 | 3.44 | 3.16-3.75 |
| A/Shiga/5/2002 | H3N2 | CI | 64.2 | 51.5-78.9 | 10.1 | 9.29-11.0 | 4.82 | 4.06-5.88 | 0.706 | 0.597-0.843 |
| A/Yamagata/1/2002 | H3N2 | CI | 56.6 | 45.5-69.1 | 14.2 | 12.2-16.6 | 7.58 | 6.07-10.1 | 1.09 | 0.981-1.22 |
| A/Yamagata/2/2002 | H3N2 | CI | 46.6 | 40.8-53.0 | 7.09 | 5.36-9.62 | 6.45 | 5.08-8.87 | 0.981 | 0.880-1.09 |
| A/Saitama/80/2003 | H3N2 | CI | 39.2 | 31.5-48.0 | 10.4 | 7.57-15.3 | 7.16 | 4.86-13.8 | 0.711 | 0.550-0.940 |
| A/Osaka/56/2004 | H3N2 | CI | 129 | 118-141 | 8.72 | 6.96-11.2 | 4.84 | 4.07-5.93 | 1.08 | 0.836-1.48 |
| A/Tokushima/1/2005 | H3N2 | CI | 131 | 117-147 | 7.54 | 5.76-11.0 | 3.42 | 3.02-3.88 | 0.925 | 0.742-1.19 |
| A/Saitama/07/2006 | H3N2 | CI | 63.3 | 52.8-75.0 | 13.0 | 11.2-15.3 | 6.04 | 5.01-7.62 | 0.885 | 0.716-1.12 |
| Mean ± SD | 114 ± 55.5 | 16.7 ± 8.62 | 7.44 ± 2.70 | 1.49 ± 0.738 | ||||||
| Type B | ||||||||||
| B/Brisbane/32/2002 | Victoria | VS | 9,890 | 8,880-11,000 | 30.5 | 26.2-35.5 | 7.78 | 6.29-9.83 | 13.6 | 12.2-15.1 |
| B/Malaysia/2506/2004 | Victoria | VS | 11,100 | 10,400-11,900 | 31.3 | 28.7-34.0 | 8.66 | 6.94-11.1 | 15.1 | 13.6-16.7 |
| B/Ohio/01/2005 | Victoria | VS | 12,800 | 11,800-13,900 | 30.0 | 27.6-32.7 | 7.86 | 6.45-9.75 | 13.6 | 11.9-15.5 |
| B/Shiga/17/2002 | Victoria | CI | 7,680 | 6,980-8,490 | 18.2 | 16.9-19.6 | 7.11 | 5.45-10.3 | 8.72 | 5.99-11.8 |
| B/Yamagata/15/2002 | Victoria | CI | 6,980 | 6,310-7,760 | 15.9 | 13.6-19.2 | 5.68 | 4.48-7.72 | 7.25 | 5.81-8.94 |
| B/Akita/9/2003 | Victoria | CI | 13,800 | 12,400-15,600 | 12.4 | 10.8-14.5 | 3.79 | 3.35-4.33 | 9.55 | 8.04-11.3 |
| B/Tokushima/1/2003 | Victoria | CI | 13,800 | 12,600-15,200 | 11.4 | 10.1-13.0 | 3.55 | 3.12-4.07 | 9.26 | 7.77-11.0 |
| B/Yamagata/145/2003 | Victoria | CI | 15,900 | 13,900-18,300 | 10.4 | 7.86-14.7 | 4.05 | 3.44-4.84 | 3.09 | 2.83-3.37 |
| B/Yamagata/398/2003 | Victoria | CI | 12,400 | 11,700-13,200 | 12.5 | 11.1-14.2 | 3.78 | 3.33-4.32 | 10.1 | 8.53-12.0 |
| B/Aichi/3/2006 | Victoria | CI | 13,800 | 12,900-14,900 | 19.3 | 16.0-24.4 | 4.85 | 4.19-5.71 | 12.4 | 11.0-14.0 |
| B/Saitama/01/2006 | Victoria | CI | 45,500 | 42,000-49,800 | 26.5 | 19.9-40.2 | 6.41 | 5.16-8.46 | 13.7 | 11.7-16.0 |
| B/Mie/1/1993 | Yamagata | VS | 6,850 | 6,150-7,610 | 31.3 | 28.2-34.8 | 12.0 | 10.6-13.6 | 15.6 | 14.2-17.1 |
| B/Shanghai/361/2002 | Yamagata | VS | 6,660 | 5,920-7,470 | 31.4 | 28.6-34.6 | 10.1 | 7.76-13.9 | 12.0 | 10.5-13.6 |
| B/Shiga/31/2002 | Yamagata | CI | 10,500 | 10,000-11,100 | 14.0 | 12.1-16.4 | 4.90 | 4.08-6.08 | 9.16 | 7.61-11.0 |
| B/Shizuoka/58/2004 | Yamagata | CI | 7,530 | 6,850-8,310 | 16.0 | 13.6-19.4 | 5.67 | 4.66-7.24 | 8.34 | 7.06-9.81 |
| B/Yamagata/85/2004 | Yamagata | CI | 7,190 | 6,610-7,830 | 18.3 | 15.4-22.7 | 6.44 | 5.25-8.33 | 8.84 | 7.51-10.4 |
| B/Yamagata/87/2004 | Yamagata | CI | 7,790 | 7,470-8,110 | 16.7 | 14.0-20.4 | 5.82 | 4.86-7.26 | 8.26 | 6.95-9.78 |
| B/Aichi/186/2005 | Yamagata | CI | 3,550 | 3,160-3,980 | 15.0 | 12.8-18.1 | 6.72 | 5.45-8.79 | 9.21 | 8.02-10.6 |
| B/Saitama/01/2005 | Yamagata | CI | 3,610 | 3,200-4,080 | 15.0 | 13.2-17.5 | 6.89 | 5.49-9.28 | 10.6 | 9.25-12.2 |
| B/Sapporo/29/2005 | Yamagata | CI | 3,600 | 3,140-4,120 | 15.4 | 13.3-18.2 | 6.91 | 5.40-9.64 | 9.62 | 8.27-11.2 |
| B/Tokushima/1/2005 | Yamagata | CI | 3,710 | 3,260-4,240 | 14.2 | 12.2-16.8 | 6.30 | 5.18-8.04 | 8.35 | 7.16-9.71 |
| B/Yamagata/1/2005 | Yamagata | CI | 3,630 | 3,190-4,140 | 13.7 | 11.9-16.2 | 6.36 | 5.11-8.45 | 9.44 | 7.86-11.3 |
| B/Yamagata/113/2005 | Yamagata | CI | 3,500 | 3,130-3,910 | 15.8 | 13.5-19.0 | 7.59 | 5.91-10.7 | 10.6 | 9.17-12.3 |
| Mean ± SD | 10,100 ± 8,670 | 18.9 ± 7.20 | 6.49 ± 2.02 | 10.3 ± 2.82 | ||||||
Means and standard deviations (SD) of NA inhibitor compound IC50s to H1N1 (11 strains), H3N2 (15 strains), and type B viruses (23 strains) are shown.
LS, laboratory strain; VS, vaccine strain; CI, clinical isolate.
Maximum and minimum IC50s of each compound against H1N1, H3N2, and type B viruses are underlined. IC50s of NA inhibitors to B viruses in boldface indicate maximum or minimum IC50s of each inhibitor to the clinical isolates. CI, confidence interval.
For the 15 strains of H3N2 viruses, R-125489 showed NA inhibitory activities with IC50s of 7.09 to 38.8 nM (mean, 16.7 nM). Zanamivir and oseltamivir carboxylate showed NA inhibitory activities with IC50s of 3.42 to 14.2 nM (mean, 7.44 nM) and 0.706 to 3.44 nM (mean, 1.49 nM), respectively. CS-8958 showed comparably weak inhibitory activities with IC50s of 39.2 to 221 nM (mean, 114 nM). R-125489 maintained potent activity against the 1968 isolate (A/Aichi/2/68) through the recent 2006 isolate.
For the 23 strains of B viruses, R-125489 showed NA inhibitory activities with IC50s of 10.4 to 31.4 nM (mean, 18.9 nM). Zanamivir and oseltamivir carboxylate showed NA inhibitory activities with IC50s of 3.55 to 12.0 nM (mean, 6.49 nM) and 3.09 to 15.6 nM (mean, 10.3 nM), respectively. CS-8958 basically showed no inhibitory activities, with IC50s of 3,500 to 45,500 nM (mean, 10,100 nM).
Though only one virus strain was checked, R-125489 again showed potent inhibitory activity with an IC50 of 11.4 nM to the H2N2 (A/Singapore/1/57) virus. The IC50s of zanamivir, oseltamivir carboxylate, and CS-8958 were 3.66, 0.925, and 128 nM, respectively.
Inhibition of the NA activity of animal influenza viruses.
The IC50s of NA inhibitors to the animal influenza virus subtypes N3 to N9 and H5N1 virus are shown in Table 2. R-125489 showed NA inhibitory activities with IC50s of 1.81 to 27.9 nM. Zanamivir and oseltamivir carboxylate showed NA inhibitory activities with IC50s of 1.40 to 11.5 nM and 1.43 to 3.65 nM, respectively. CS-8958 showed either no or weak inhibitory activities with IC50s of 142 to 1,140 nM. R-125489 maintained potent activity against animal influenza virus subtypes N3 to N9 and to the H5N1 virus.
TABLE 2.
IC50s and 95% CIs of NA activities against animal influenza virusesb
| Influenza virus | Subtype | CS-8958
|
R-125489
|
Zanamivir
|
Oseltamivir carboxylate
|
||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | 95% CI | IC50 (nM) | 95% CI | IC50 (nM) | 95% CI | IC50 (nM) | 95% CI | ||
| A/R(duck/Mongolia/54/01- duck/Mongolia/47/01)a | H5N1 | 276 | 254-300 | 4.54 | 3.91-5.37 | 2.99 | 2.25-4.20 | 2.17 | 1.94-2.43 |
| A/duck/Hokkaido/84/2002 | H5N3 | 456 | 413-502 | 13.3 | 11.6-15.6 | 3.65 | 2.63-5.57 | 2.94 | 2.80-3.10 |
| A/turkey/Ontario/6,118/1968 | H8N4 | 420 | 378-471 | 7.08 | 6.55-7.71 | 5.58 | 4.35-7.71 | 3.65 | 2.99-4.37 |
| A/duck/Alberta/60/1976 | H12N5 | 1,140 | 1,020-1,270 | 2.34 | 2.24-2.44 | 1.83 | 1.49-2.35 | 1.43 | 1.31-1.55 |
| A/duck/England/1/1956 | H11N6 | 262 | 242-282 | 25.5 | 24.9-26.3 | 8.57 | 8.11-9.06 | 2.81 | 2.70-2.92 |
| A/seal/Massachusetts/1/1980 | H7N7 | 142 | 121-165 | 27.9 | 25.9-30.1 | 11.5 | 10.5-12.6 | 2.56 | 2.42-2.72 |
| A/duck/Ukraine/1/1963 | H3N8 | 1,050 | 1,010-1,090 | 1.81 | 1.77-1.84 | 1.40 | 1.24-1.61 | 2.12 | 1.93-2.34 |
| A/duck/Memphis/546/1974 | H11N9 | 356 | 313-403 | 6.81 | 6.51-7.14 | 3.31 | 3.11-3.53 | 1.70 | 1.66-1.73 |
Reassortant virus generated from A/duck/Mongolia/54/01 (H5N2) and A/duck/Mongolia/47/01 (H7N1).
Maximum and minimum IC50s are underlined for each compound. CI, confidence interval.
Inhibition of the NA activity of oseltamivir-resistant influenza viruses isolated from patients.
The IC50s of NA inhibitors to the oseltamivir-resistant H1N1, H3N2, and type B influenza viruses are shown in Table 3. The IC50s of oseltamivir carboxylate against the oseltamivir-resistant H1N1 virus with the H274Y mutation and against the H3N2 viruses with the R292K, E119V, and N294S mutations were 755 nM (mutant/wild-type IC50 ratio, 330), 10,400 nM (ratio, 8,400), 140 nM (ratio, 79), and 37.2 nM (ratio, 32), respectively. On the other hand, the IC50 ratios of R-125489 and zanamivir to the H1N1 virus with the H274Y mutation and the H3N2 viruses with the R292K, E119V, and N294S mutations to their corresponding wild types were 1.9, 0.69, 0.69, and 2.8 (R-125489), respectively, and 1.1, 1.4, 0.72, and 1.7 (zanamivir), respectively.
TABLE 3.
IC50s, 95% CIs, and mutant/wild-type ratios of NA activities against oseltamivir-resistant influenza virusesb
| Influenza virus | Virus type or subtype | Mutation in NAa | R-125489
|
Zanamivir
|
Oseltamivir carboxylate
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | 95% CI | Ratio | IC50 (nM) | 95% CI | Ratio | IC50 (nM) | 95% CI | Ratio | |||
| A/Yokohama/67/2006 (clone 1) | H1N1 | WT | 3.03 | 2.75-3.35 | 2.70 | 2.53-2.90 | 2.28 | 2.12-2.43 | |||
| 1.9 | 1.1 | 330 | |||||||||
| A/Yokohama/67/2006 H274Y (clone 11) | H1N1 | H274Y | 5.62 | 4.75-6.89 | 3.05 | 2.88-3.23 | 755 | 727-784 | |||
| A/Kawasaki/IMS22A-954/2003 | H3N2 | WT | 15.4 | 13.4-18.0 | 8.29 | 7.87-8.72 | 1.25 | 1.09-1.45 | |||
| 0.69 | 1.4 | 8,400 | |||||||||
| A/Kawasaki/IMS22B-955/2003 | H3N2 | R292K | 10.6 | 9.83-11.4 | 11.2 | 9.59-13.0 | 10,400 | 9,600-11,400 | |||
| A/Yokohama/IMS9A-2029/2003 | H3N2 | WT | 19.2 | 15.9-24.2 | 10.7 | 10.1-11.4 | 1.78 | 1.76-1.80 | |||
| 0.69 | 0.72 | 79 | |||||||||
| A/Yokohama/IMS9B-2050/2003 | H3N2 | E119V | 13.2 | 11.7-15.0 | 7.71 | 7.58-7.85 | 140 | 120-165 | |||
| A/Kawasaki/MS31A-1030/2002 | H3N2 | WT | 13.4 | 11.8-15.3 | 7.82 | 7.57-8.10 | 1.18 | 1.07-1.31 | |||
| 2.8 | 1.7 | 32 | |||||||||
| A/Kawasaki/MS31B-1206/2002 | H3N2 | N294S | 37.3 | 33.9-41.2 | 13.5 | 12.0-15.4 | 37.2 | 34.7-39.7 | |||
| B/Yokohama/UT2203/2005 | B | D198N | 48.9 | 41.7-58.7 | — | 33.6 | 31.6-35.8 | — | 40.5 | 36.8-44.6 | — |
| B/Yokohama/UT3318/2005 | B | I222T | 30.2 | 27.6-33.0 | — | 20.0 | 16.2-26.4 | — | 68.8 | 63.8-74.1 | — |
Amino acids at left mutated to the amino acids at right at the indicated residue numbers, based on N2 NA numbering. Amino acids are described using one-letter symbols. WT, wild type.
Mutant/wild-type IC50 ratios are shown. CI, confidence interval; —, not applicable.
For type B virus D198N and I222T mutants, NA inhibitors showed IC50s of 48.9 nM and 30.2 nM (R-125489) and 33.6 nM and 20.0 nM (zanamivir) and 40.5 nM and 68.8 nM (oseltamivir carboxylate), respectively. Because the mutants’ corresponding wild-type viruses were not obtained from the patients who generated the mutants, the mutant/wild-type IC50 ratios were not calculated.
In vivo efficacy of R-125489.
The IC50 of NA inhibition of R-125489 to A/PR/8/34 virus was about two times weaker than that of zanamivir. We then compared the in vivo efficacy of R-125489 with that of zanamivir in a mouse influenza virus infection model. The compounds were intranasally administered three times, at 0.2 μmol/kg per dose, 4 h before infection and again at 4 h and 18 h postinfection (p.i.) with the A/PR/8/34 virus (500 PFU/mouse), and the survival of the mice was monitored for 20 days. While all the control mice died by day 6 p.i., all the mice that were administered zanamivir died by day 10 p.i., which showed a significantly prolonged survival effect (Fig. 2). Under these experimental conditions, R-125489 provided a higher survival rate than zanamivir did, and 20% of the mice that received R-125489 survived for as long as 20 days p.i., but the difference in survival rate between R-125489 and zanamivir was not statistically significant (P = 0.0732).
In vivo efficacy of esterified forms of R-125489.
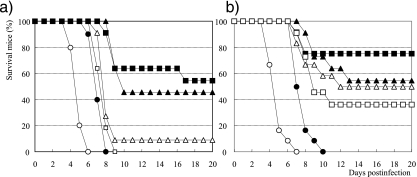
The in vivo prolonged-survival effect of R-125489 was not much different from that of zanamivir. Next, we evaluated the efficacy of acylated R-125489 compounds with various carbon chain lengths at position 3. The mice were infected with 1,500 PFU of A/PR/8/34 and (at 4 h before and 4 h and 17 h p.i.) were administered three intranasal doses at 0.3 μmol/kg of the compounds. Under these experimental conditions, in which all control mice died between days 6 and 7 p.i. and all mice given zanamivir died between days 8 and 10 p.i., the prodrugs provided prolonged survival effects in accordance with the chain length. Mice that received CS-8958, an esterified form with an 8-carbon chain, had the highest survival rate, and more than half the mice given CS-8958 survived for 20 days p.i. (Fig. 3a and b). All mice infected with 500 PFU of A/PR/8/34 survived after three administrations of 0.2 μmol/kg of CS-8958 (Fig. 2).
FIG. 3.
In vivo efficacy of the prodrug of R-125489 with acyl chains of various lengths in a mouse influenza virus infection model. Influenza A virus A/PR/8/34-infected (1,500 PFU, 0 h) mice given 0.3 μmol/kg of R-125489-C8 (CS-8958, filled squares), R-125489-C10 (filled triangles), R-125489-C6 (open triangles), R-125489-C4 (open squares), zanamivir (filled circles), and saline (open circles) (a) or R-125489-C8 (CS-8958, filled squares), R-125489-C10 (filled triangles), R-125489-C14 (open triangles), R-125489-C16 (open squares), zanamivir (filled circles), and saline (open circles) (b) at 4 h before infection and 4 h and 17 h p.i. The number of surviving mice was monitored for 20 days p.i. (n = 10 to 12).
Efficacy of prophylaxis administration.
CS-8958 or zanamivir was intranasally administered to mice once at a dose of 0.5 μmol/kg on days 10, 7, 4, and 1 before infection with A/PR/8/34, and the surviving mice were monitored (Fig. 4). No significant differences in efficacy were observed between the groups administered zanamivir at days 10, 7, and 4 before infection (P values of 0.4279, 0.5820, and 0.0579, respectively). Only the group that was given zanamivir at day 1 before infection showed significant benefit (P = 0.0151). On the other hand, all the groups receiving CS-8958 on days 10, 7, 4, and 1 before infection showed significantly prolonged survival (P values of 0.0230 for mice dosed at day −10 and <0.0001 for the other three groups).
DISCUSSION
R-125489, a new NA inhibitor, was discovered, and in this study, its NA inhibitory activities against various influenza viruses including oseltamivir-resistant viruses are reported. During this study, we also found that 3-(O)-octanoyl R-125489 (CS-8958) showed a prolonged survival effect in a mouse influenza virus infection model.
R-125489 is a compound which is structurally related to zanamivir, and the IC50s of the NA inhibitory activity against the influenza viruses tested were generally weaker than those of zanamivir. The NA inhibitory activity of R-125489 was weaker than that of oseltamivir carboxylate, as well. In particular, in terms of activity against the H3N2 strains, the IC50s of the NA inhibition of R-125489 were about 10 times weaker than those of oseltamivir carboxylate. In a mouse influenza virus A/PR8/34 infection model, the survival rate of mice in the R-125489 administration group was higher than that of mice in the zanamivir administration group (Fig. 2), but the difference was not significant (P = 0.0732). The in vivo prolonged survival effect may not directly reflect the NA inhibitory activity but may reflect viral replication inhibitory activity because the 50% effective concentration of R-125489 in inhibiting A/PR/8/34 replication was about five times stronger than that of zanamivir, as determined by a plaque reduction assay (data not shown).
A pandemic caused by a new influenza virus is always a threat to humans. Highly pathogenic avian influenza H5N1 virus is thought to be the next pandemic virus, but a virus with other subtypes could possibly cause a pandemic. R-125489 inhibits the viral NA of the N3 to N9 strains, as well as those of the H5N1 (Table 2) and H2N2 (Table 1) strains. R-125489 and CS-8958 could be candidate drugs for the treatment and prevention of pandemic influenza.
Currently, oseltamivir is used mainly for influenza treatment, but the generation and/or circulation of oseltamivir-resistant mutants in humans has become a major concern (6, 8, 11, 12, 13). We were provided with one H1N1 mutant isolated during an influenza surveillance and three H3N2 mutants isolated from infected patients (11). While oseltamivir carboxylate shows IC50 ratios of 32 to 8,400 to the H274Y (H1N1), R292K, E119V, and N294S (H3N2) mutants, the IC50 ratios of R-125489 and zanamivir for these strains are 0.69 to 2.8 and 0.72 to 1.7, respectively. This suggested that R-125489 and zanamivir essentially maintained their activities against the oseltamivir-resistant mutants. A crystal structure analysis of R-125489-NA was not performed, but R-125489 is structurally related to zanamivir. Therefore, it is anticipated that the ratios of the IC50s of R-125489 and zanamivir against resistant virus compared to those against wild-type virus will be similar.
The B virus mutants were isolated from infected patients (6). Since the mutants’ corresponding wild-type viruses were not obtained, the IC50 ratios could not be calculated. The IC50s of oseltamivir carboxylate to the clinical isolates obtained between 2002 and 2006 ranged from 3.09 to 13.7 nM (Table 1). As the IC50s of oseltamivir carboxylate to the D198N and I222T mutants were 40.5 and 68.8 nM, respectively, these mutants may be resistant to oseltamivir carboxylate, and the ratios are not that large compared to those for A virus mutants. On the other hand, the IC50s of R-125489 and zanamivir to the clinical isolates were 10.4 to 26.5 nM and 3.55 to 7.59 nM (Table 1), respectively, and were 48.9 and 30.2 nM (R-125489) and 33.6 and 20.0 nM (zanamivir) to the D198N and I222T mutants, respectively. Therefore, these mutants may not be resistant—or may be less resistant—to R-125489 and zanamivir.
R-125489 did not inhibit other NAs such as those of Vibrio cholerae, Clostridium perfringens, and Newcastle disease virus (IC50s of >100 μM to NAs of all these organisms) (data not shown). In addition, R-125489 did not show cytotoxicity to MDCK, HeLa, and MOLT-4 cell lines [50% cytotoxicity concentration of >100 μg/ml (290 μM) as determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) standard colorimetric assay; data not shown]. We believe that R-125489 is a specific inhibitor of influenza virus NA and is a good candidate for an anti-influenza agent. However, the efficacy of R-125489 administered intranasally in a mouse influenza virus infection model showed only slight improvement over that of zanamivir (Fig. 2).
Next, we made an effort to improve the efficacy of R-125489. Esterified R-125489 compounds with various acyl chains at position 3 were made, and their efficacies in a mouse influenza virus infection model were evaluated. The esterified R-125489 compound with chains of more than 6 carbons showed better efficacy than zanamivir did, and R-125489-C8 (CS-8958) was the best compound in terms of its life-prolonging effect (Fig. 3). CS-8958 itself showed very weak or no inhibition of viral NA. Interestingly, the differences between the IC50s of CS-8958 and R-125489 to the H3N2 and H2N2 strains were around 10-fold, but the difference to the H1N1 strain and B viruses were about 100- to 1,000-fold. The interaction of the acyl chain with the NA may be different among the NA subtypes and the virus types.
The reason for improvement in efficacy resulting from the acylation of R-125489 is not clear, but the long retention of the compound in the lungs may be a possible mechanism of improvement in the drug's efficacy. In fact, a single intranasal administration of CS-8958 to mice even 10 days before infection showed a life-prolonging effect, while the same effect was not shown with zanamivir (Fig. 4). It has been reported that some dimers of zanamivir show dissociation constant (Kd) values that are 0.5 to 1.5 orders of magnitude greater than those for zanamivir for NA inhibition activity and suggested that the high antiviral activity of the zanamivir dimers is a result of their ability to cross-link and aggregate virus particles. The dimers also showed long-lasting protective activities in a mouse influenza virus infection model (14, 15). CS-8958 itself did not show a strong inhibition of NA, but it is suggested that intranasally administered CS-8958 works as a long-acting NA inhibitor in a mouse influenza virus infection model. We have reported (16, 19) that CS-8958 (described as R-118958 in that article) was quickly metabolized to R-125489 in the lungs after intranasal administration of CS-8958 to mice and that it was retained as the metabolite for a long time. On the other hand, intranasally administered R-125489 was quickly removed from the lungs. Therefore, CS-8958 might work as a prodrug of R-125489 (unpublished pharmacokinetic data). The long-retention mechanism of CS-8958 might be different from that of dimers of zanamivir. The dimers may be retained in their original forms in lungs and not work as prodrugs (15).
In contrast to the regimens of currently available drugs, it is expected that a single inhalation of CS-8958 might be enough to treat influenza and that a once weekly inhalation might be enough for prevention. This dose regimen of CS-8958 allows for better compliance by influenza patients and is a desirable characteristic for prophylaxis, especially for the measures implemented against pandemic influenza. A phase 2 clinical trial using a single inhalation of CS-8958 is currently under way to determine the drug's potential as a long-acting NA inhibitor for the treatment of seasonal influenza. In addition, CS-8958 is expected to show anti-influenza activity against oseltamivir-resistant viruses, as well as against the predicted pandemic influenza viruses.
Acknowledgments
We thank T. Odagiri and M. Obuchi (National Institute of Infectious Diseases, Japan) for providing the laboratory and vaccine strains, H. Kida and Y. Sakoda (the Graduate School of Veterinary Medicine, Hokkaido University) for the animal viruses, C. Kawakami (Yokohama City Institute of Health) for the resistant mutants, and Y. Kawaoka and M. Kiso (Division of Virology, Department of Microbiology and Immunology, the Institute of Medical Science, the University of Tokyo) for the resistant mutants. We also thank Y. Ohuchi (Shiga Prefectural Institute of Public Health), K. Mizuta (Yamagata Prefectural Institute of Public Health), S. Shimada and Y. Kikuchi (Saitama Institute of Public Health), M. Hata (Aichi Prefectural Institute of Public Health), K. Taira (Okinawa Prefectural Institute of Health and Environment), T. Kase (Osaka Prefectural Institute of Public Health), Y. Yamamoto (Tokushima Prefectural Institute of Public Health and Environmental Sciences), H. Saito (Akita Research Center for Public Health and Environment), Shizuoka Institute of Environment and Hygiene, and M. Kikuchi (Sapporo City Institute of Public Health) for providing the clinical isolates. We also thank Takeshi Honda (Medicinal Chemistry Research Laboratories I, Daiichi Sankyo Co., Ltd.) for synthesizing the compounds.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Bantia, S., A. A. Ghate, S. L. Ananth, Y. S. Babu, G. M. Air, and G. M. Walsh. 1998. Generation and characterization of a mutant of influenza A virus selected with the neuraminidase inhibitor BCX-140. Antimicrob. Agents Chemother. 42:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bright, R. A., D. K. Shay, B. Shu, N. J. Cox, and A. I. Klimov. 2006. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 295:891-894. [DOI] [PubMed] [Google Scholar]
- 3.Bright, R. A., D. Shay, J. Bresee, A. Klimov, N. Cox, and J. Ortiz. 2006. High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents—United States, 2005-06 influenza season. MMWR Morb. Mortal. Wkly. Rep. 55:44-46. [PubMed] [Google Scholar]
- 4.Fiore, A. E., D. K. Shay, P. Haber, J. K. Iskander, T. M. Uyeki, G. Mootrey, J. S. Bresee, and N. J. Cox. 2007. Prevention and control of influenza recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 56:1-54. [PubMed] [Google Scholar]
- 5.Gubareva, L. V. 2004. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 103:199-203. [DOI] [PubMed] [Google Scholar]
- 6.Hatakeyama, S., N. Sugaya, M. Ito, M. Yamazaki, M. Ichikawa, K. Kimura, M. Kiso, H. Shimizu, C. Kawakami, K. Koike, K. Mitamura, and Y. Kawaoka. 2007. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA 297:1435-1442. [DOI] [PubMed] [Google Scholar]
- 7.Hay, A. J., A. J. Wolstenholme, J. J. Skehel, and M. H. Smith. 1985. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 4:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden, F. G. 2006. Antiviral resistance in influenza viruses—implications for management and pandemic response. N. Engl. J. Med. 354:785-788. [DOI] [PubMed] [Google Scholar]
- 9.Honda, T., T. Masuda, S. Yoshida, M. Arai, Y. Kobayashi, and M. Yamashita. 2002. Synthesis and anti-influenza virus activity of 4-guanidino-7-substituted Neu5Ac2en derivatives. Bioorg. Med. Chem. Lett. 12:1921-1924. [DOI] [PubMed] [Google Scholar]
- 10.Honda, T., T. Masuda, S. Yoshida, M. Arai, S. Kaneko, and M. Yamashita. 2002. Synthesis and anti-influenza virus activity of 7 derivatives related to zanamivir. Bioorg. Med. Chem. Lett. 12:1925-1928. [DOI] [PubMed] [Google Scholar]
- 11.Kiso, M., K. Mitamura, Y. Sakai-Tagawa, K. Shiraishi, C. Kawakami, K. Kimura, F. G. Hayden, N. Sugaya, and Y. Kawaoka. 2004. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364:759-765. [DOI] [PubMed] [Google Scholar]
- 12.Lackenby, A., O. Hungnes, S. G. Dudman, A. Meijer, W. J. Paget, A. J. Hay, and M. C. Zambon. 2008. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 13:1-2. [DOI] [PubMed] [Google Scholar]
- 13.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. L. Nguyen, N. D. Pham, H. H. Ngyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature (London) 437:1108. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald, S. J., R. Cameron, D. A. Demaine, R. J. Fenton, G. Foster, D. Gower, J. N. Hamblin, S. Hamilton, G. J. Hart, A. P. Hill, G. G. Inglis, B. Jin, H. T. Jones, D. B. McConnell, J. McKimm-Breschkin, G. Mills, V. Nguyen, I. J. Owens, N. Parry, S. E. Shanahan, D. Smith, K. G. Watson, W. Y. Wu, and S. P. Tucker. 2005. Dimeric zanamivir conjugates with various linking groups are potent, long-lasting inhibitors of influenza neuraminidase including H5N1 avian influenza. J. Med. Chem. 48:2964-2971. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald, S. J., K. G. Watson, R. Cameron, D. K. Chalmers, D. A. Demaine, R. J. Fenton, D. Gower, J. N. Hamblin, S. Hamilton, G. J. Hart, G. G. Inglis, B. Jin, H. T. Jones, D. B. McConnell, A. M. Mason, V. Nguyen, I. J. Owens, N. Parry, P. A. Reece, S. E. Shanahan, D. Smith, W. Y. Wu, and S. P. Tucker. 2004. Potent and long-acting dimeric inhibitors of influenza virus neuraminidase are effective at a once-weekly dosing regimen. Antimicrob. Agents Chemother. 48:4542-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai, N., N. Yamamura, M. Hoshi, M. Oitate, N. Kobayashi, T. Hirota, M. Kazui, and K. Kawai. 2003. R-118958, a unique anti-influenza agent: pharmacokinetics and metabolism after intranasal/intratracheal administration to rats and mice. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1833.
- 17.Ryan, D. M., J. Ticehurst, M. H. Dempsey, and C. R. Penn. 1994. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase). Antimicrob. Agents Chemother. 38:2270-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright, P. F., and R. G. Webster. 2001. Orthomyxoviruses, p. 1534-1579. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 19.Yamashita, M. 2004. R-118958, a unique anti-influenza agent showing high efficacy for both prophylaxis and treatment after a single administration: from the in vitro stage to phase I study. Int. Congr. Ser. 1263:38-42. [Google Scholar]