Abstract
Accumulation of antiviral nucleotides in renal proximal tubules is controlled by their basolateral uptake via the human renal organic anion transporters type 1 (hOAT1) and 3 (hOAT3) and apical efflux via the multidrug resistance protein 4 (MRP4). GS-9148 is a novel ribose-modified nucleotide human immunodeficiency virus (HIV) reverse transcriptase inhibitor, and its oral prodrug GS-9131 is currently being evaluated in the clinic as an anti-HIV agent. To assess the potential of GS-9148 for nephrotoxicity, its mechanism of renal transport, cytotoxicity, and renal accumulation were explored in vitro and in vivo. In comparison with the acyclic nucleotides cidofovir, adefovir, and tenofovir, GS-9148 showed 60- to 100-fold lower efficiency of transport (Vmax/Km) by hOAT1 and was 20- to 300-fold less cytotoxic in cells overexpressing hOAT1, indicating its lower hOAT1-mediated intracellular accumulation and reduced intrinsic cytotoxicity. GS-9148 was also relatively inefficiently transported by hOAT3. Similar to acyclic nucleotides, GS-9148 was a substrate for MRP4 as evidenced by its reduced intracellular retention in cells overexpressing the efflux pump. Consistent with these molecular observations, GS-9148 was inefficiently taken up by fresh human renal cortex tissue in vitro and showed a limited accumulation in kidneys in vivo following oral administration of [14C]GS-9131 to dogs. Compared to acyclic nucleotide analogs, GS-9148 was also found to have lower net active tubular secretion in dogs. Collectively, these results suggest that GS-9148 exhibits a low potential for renal accumulation and nephrotoxicity.
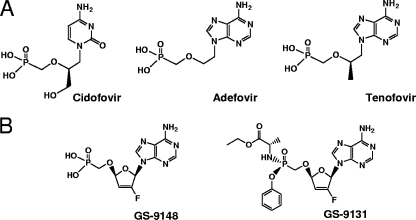
The acyclic nucleotide analogs tenofovir, adefovir, and cidofovir (Fig. 1A) have emerged as a novel class of clinically effective antiviral agents used for the treatment of various viral infections, including human immunodeficiency virus (HIV) (21, 24), hepatitis B virus (13), and cytomegalovirus (19). Drug-related renal adverse effects manifested by changes in the function of renal proximal tubules have been identified in a subset of patients treated with certain nucleotide regimens (17, 23, 28). Acyclic nucleotides are secreted renally by a combination of glomerular filtration and active tubular secretion (7); their accumulation in kidney tissue has been detected in several animal species (9, 10, 20), and subsequent molecular studies have demonstrated the ability of human renal organic anion transporters types 1 and 3 (hOAT1 and hOAT3) to efficiently transport these nucleotides (3, 15, 31). Both hOAT1 and hOAT3 are selectively expressed in the basolateral membrane of renal proximal tubule cells (22), indicating that these transporters play an important role in the renal secretion of nucleotides and, in certain circumstances, mediate their accumulation in proximal tubules, which may subsequently lead to renal toxicity. In addition, it has been recently established that tenofovir and adefovir are substrates for multidrug resistance protein 4 (MRP4), but not MRP2 or P-glycoprotein efflux pumps (4, 16, 25). Functional MRP4 is present in the apical membrane of renal proximal tubules and mediates elimination of its substrates from proximal tubule cells into urine (32). Therefore, in addition to being a part of the nucleotide active tubular secretion pathway, MRP4 activity serves a nephroprotective role by reducing the tubular accumulation of nucleotide analogs.
FIG. 1.
(A) Structures of the acyclic nucleotide analogs cidofovir, adefovir, and tenfovir. (B) Structures of a ribose-modified nucleotide analog, GS-9148, and its phosphonoamidate prodrug, GS-9131.
GS-9148 (phosphonomethoxy-2′-fluoro-2′,3′-dideoxydidehydroadenosine; Fd4AP) (Fig. 1B) is a novel nucleotide HIV reverse transcriptase inhibitor with a modified ribose-like sugar moiety and a phosphonate linkage (5). Its mono-amidate prodrug GS-9131 substantially increases the cellular permeability of GS-9148 and is readily converted to the parent nucleotide inside cells (26). GS-9131 exhibits potent antiretroviral activity in vitro against a range of HIV type 1 (HIV-1) strains, including clinical isolates of different subtypes, and shows a favorable in vivo pharmacokinetic profile, effectively delivering both the parent nucleotide GS-9148 and its active diphosphate metabolite into peripheral blood mononuclear cells (PBMCs) (5, 26). Importantly, the parent nucleotide GS-9148 shows a favorable resistance profile and retains its activity against a wide range of nucleoside-resistant HIV-1 variants, including viruses with multiple M184V, K65R, L74V, and thymidine analog mutations in reverse transcriptase (5). Because of these favorable pharmacological properties, GS-9131 is currently being explored as a novel antiretroviral agent for the treatment of HIV infection.
Similar to cidofovir, adefovir, and tenofovir, GS-9148 carries a negative charge under physiological conditions and, hence, it has the potential to accumulate in renal proximal tubules via hOAT1- and hOAT3-mediated uptake. The goal of this study was to assess the interaction of GS-9148 with the renal uptake and efflux transporters and to understand more broadly the potential of GS-9148 to actively accumulate in renal proximal tubules in vivo, a process that may be prerequisite to renal toxicity.
MATERIALS AND METHODS
Compounds and reagents.
GS-9148 was synthesized as previously described (2). Tenofovir, adefovir, and cidofovir were provided by the Process Chemistry Department, Gilead Sciences. Stock solutions of all nucleotides were prepared in water and adjusted to pH 7.0. Probenecid was purchased from Sigma (St. Louis, MO) and dissolved in dimethyl sulfoxide. [14C]cidofovir (HPMPC; specific activity, 55 mCi/mmol), [3H]adefovir (PMEA; specific activity, 9.0 Ci/mmol), [3H]tenofovir (PMPA; specific activity, 11.0 Ci/mmol), and [3H]tenofovir disoproxil (bis-POC-PMPA; specific activity, 4.5 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA). Custom synthesis of [14C]GS-9148 and [14C]GS-9131 was performed by Moravek Biochemicals according to a protocol developed by Gilead Sciences. The efflux inhibitor MK-571 was purchased from Cayman Chemical Company (Ann Arbor, MI).
Cells.
The generation, characterization, and maintenance of Chinese hamster ovary cells stably transfected with hOAT1 (CHOhOAT1) and control cells transfected with empty pIRESneo3 expression vector (CHOpIRES) (BD Biosciences Clontech, Mountain View, CA) have been described previously (15). The cells were passaged twice a week and maintained in phenol red-free RPMI 1640 medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1 mg/ml G-418 (Sigma). To generate BHKhOAT3 cells, cDNA encoding hOAT3 (GenBank accession no. 042505) was amplified by PCR from a human kidney cDNA library (Stratagene, La Jolla, CA) and cloned into a pIRESneo3 expression vector by using EcoRI/NheI restriction sites. The resulting plasmid was transfected into BHK-21 cells (ATCC, Manassas, VA) using GeneJammer lipid transfection reagent (Stratagene), and stably transfected clones were selected in the presence of 1 mg/ml G-418. Following the isolation and expansion of clones, approximately 20 clones were screened for the probenecid-sensitive uptake of estrone sulfate and tenofovir. The clone with the highest functional expression of hOAT3 was used for transport studies. Control BHKpIRES cells were generated by stable transfection of empty pIRESneo3 expression vector into BHK-21 cells. Cells were passaged twice a week for a maximum of 12 passages in a phenol red-free minimal essential medium (Gibco Invitrogen) supplemented with 10% fetal bovine serum and 1 mg/ml G-418.
hOAT1 and hOAT3 transport assays.
The hOAT1-specific transport assay was carried out in 12-well plates with CHOhOAT1 cells seeded 48 h before each experiment at a density of 2 × 105 cells/well. On the day of the experiment, growth medium was aspirated and the cells were washed with phosphate-buffered saline. BHKhOAT3 cells were seeded into BioCoat fibronectin-coated 12-well plates (BD Biosciences) 24 h before the transport assay at a density of 5 × 105 cells/well. CHOhOAT1 and BHKhOAT3 cells were incubated with specified concentrations of radiolabeled substrates at 37°C in Waymouth buffer (135 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 0.8 mM MgSO4, 28 mM glucose, and 13 mM HEPES, pH 7.2). In parallel, incubations under identical conditions were carried out with control CHOpIRES or BHKpIRES cells. Probenecid (500 μM) was used as an inhibitor of hOAT-mediated uptake to establish the specificity of transport processes. At the end of each incubation, cells were washed three times with ice-cold phosphate-buffered saline (2 ml/well) and lysed with 0.3% Triton X-100 (1 ml/well) for 15 min at room temperature. Cellular uptake was determined by measuring the radioactivity signal in each cell lysate after mixing with 5 ml of Ready-Safe scintillation fluid (Beckman Instruments, Fullerton, CA). Kinetics of hOAT1- and hOAT3-mediated uptake were determined in the transporter-expressing cells across an appropriate range of concentrations for each tested substrate. Various concentrations of radiolabeled substrates were incubated with CHOhOAT1 and BHKhOAT3 for 20 and 120 min, respectively. Cells were processed as described above and the values of kinetic constants (Km and Vmax) were calculated according to the Michaelis-Menten algorithm using Prism software (GraphPad, San Diego, CA). Kinetic constants were determined in two independent experiments and are presented as the mean value ± standard deviation.
MRP4 efflux assay.
MRP4-mediated efflux of nucleotides was assessed in HEK-293T cells transiently expressing the transporter as described previously (4). Briefly, cells were transfected with empty pcDNA3.1 plasmid (control samples) or pcDNA3.1 containing the human MRP4 gene (MRP4 samples) using Lipofectamine 2000 and seeded into 12-well poly-d-lysine-coated plates. The next day, cells were preloaded with either with 1 μM [3H]tenofovir DF (Moravek Biochemicals, Brea, CA) or 5 μM [14C]GS-9131 (Moravek Biochemicals) for 2 h under ATP-depleting conditions, washed, and supplemented with fresh medium with or without the efflux inhibitor MK-571 (30 μM) (4). After additional incubation for 90 min, supernatants were harvested and cell cultures were washed and lysed with 0.4% Triton X-100. Samples were analyzed for radioactivity, and the percentages of compound appearing in cell culture supernatant and remaining in cells were calculated relative to the amount of compound present in preloaded cells at the beginning of incubation.
Cytotoxicity assays.
CHOpIRES and CHOhOAT1 cells were seeded in parallel into 96-well plates at a density of 3 × 103 cells/well. After 24 h, serial dilutions of the tested drugs were added in triplicate and the cells were incubated for an additional 120 h. At the end of the incubation, cell viability was determined using a luminiscence-based Cell-Titer Glo assay (Promega, Madison, WI) according to the manufacturer's protocol. Aliquots (100 μl) of medium were removed from each sample well and replaced by 100 μl of Cell-Titer Glo reagent. The generated luminiscence signal was quantified using a Victor V3 luminiscence plate reader (Perkin-Elmer, Wellesley, MA). Cell viability was expressed as a percentage of the signal from untreated samples (0% cytotoxicity). The concentration of each drug that reduced cell viability by 50% (CC50) was determined by nonlinear regression using Prism software.
In vitro accumulation in human renal tissue.
Precision cut slices from fresh human renal cortex tissue were prepared and their treatment was conducted at Vitron, Inc. (Tucson, AZ). The tissue was received from a procurement agency with a donor consent according to accepted medical and ethical standards as defined in the Uniform Anatomical Gift Act. Renal tissue used in the described experiments was not suitable for human transplantation or therapy. Prepared slices were incubated in a transport medium containing 5 μM [3H]tenofovir or 5 μM [14C]GS-9148 and, where indicated, 500 μM probenecid was added. Samples were gently agitated for 10 and 30 min at 37°C, washed three times with ice-cold phosphate-buffered saline, frozen at −70°C, and shipped to Gilead Sciences. Upon receiving the samples, each tissue slice was lysed overnight in 0.5 ml of 0.5 N NaOH at 37°C, followed by neutralization with 0.125 ml of 2.0 N HCl. The amount of substrate taken up by the renal tissue was quantified by scintillation counting of tissue lysates upon their mixing with 5.0 ml of Ready-Safe scintillation fluid (Beckman Instruments, Fullerton, CA). Active probenecid-sensitive uptake into tissue slices was calculated by subtracting the amounts of tissue-associated substrate detected in the presence and absence of probenecid.
In vivo renal distribution and urinary clearance of GS-9148 and its metabolites.
Studies were carried out in purebred male beagle dogs by Covance Laboratories, Inc. (Madison, WI). All procedures in the study were in compliance with the Animal Welfare Act Regulations, and the study protocol was reviewed by the Institutional Animal Care and Use Committee. Six dogs were administered a target dose of 3.0 mg/kg [14C]GS-9131 (10 μCi/kg) formulated as a solution in 50 mM citrate in water (pH 2.2). All animals were fasted overnight and up to approximately 4 h postdosing. The compound solution was administered by oral gavage, and each gavage tube was washed with 5 ml of 50 mM citrate prior to withdrawal. Kidney tissue was taken from three dogs at 24 h postdosing. The tissue was excised, rinsed with saline and blotted dry, weighed, and placed on wet ice. In the other three dogs, urine was collected into plastic containers surrounded by dry ice over the time periods of 0 to 8 and 8 to 24 h postdosing. Urine and kidney tissue (following combustion) were analyzed by liquid scintillation counting. Concentrations were converted to μg equivalents of GS-9131 per gram of sample. The proportion of urinary metabolites accounted for by GS-9148 was determined in pooled urine samples from 0 to 24 h by using liquid chromatography coupled to a 500 Series in-line radioactivity detector (Packard, Meriden, CT). Metabolites were separated using a Luna C18, 3-μm, 100-mm by 4.6-mm (internal diameter) column (Phemonex, Torrance, CA), a flow rate of 1.3 ml/minute, with a buffer of 20 mM ammonium formate in water (pH 2.8) and a multistage linear gradient over 65 minutes from 1 to 50% acetonitrile. Renal clearance (CLr) was estimated using the equation CLR = (amount of GS-9148 in urine over 24 h/plasma GS-9148 exposure over 24 h)/body weight.
RESULTS
Transport of GS-9148 by hOAT1 and hOAT3.
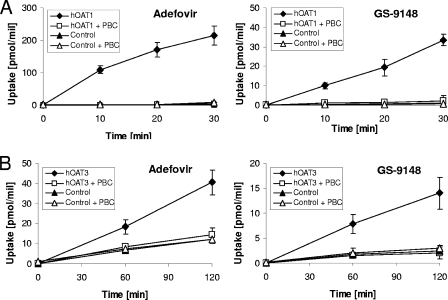
Experiments conducted in cells stably expressing functional hOAT1 or hOAT3 indicated that GS-9148 is a substrate for both of these renal transporters. Data presented in Fig. 2 show that the accumulation of GS-9148 was significantly enhanced in cells expressing either hOAT1 (CHOhOAT1) or hOAT3 (BHKhOAT3) relative to corresponding control cells transformed with empty expression vectors (CHOpIRES or BHKpIRES). Incubation of transporter-expressing cells in the presence of the organic anion transport inhibitor probenecid reduced the intracellular accumulation of GS-9148 to levels similar to those in control cells lacking the transporters. An identical pattern of cellular accumulation was observed with adefovir, an established substrate for both transporters (15, 31). At a 10 μM concentration, the hOAT1- and hOAT3-specific transport of GS-9148 was approximately 13- and 3-fold less efficient, respectively, compared to that of adefovir under the same conditions.
FIG. 2.
Transport of GS-9148 and adefovir by hOATs. (A) hOAT1. [3H]adefovir and [14C]GS-9148 at a 10 μM concentration were incubated with CHOhOAT1 cells and CHOpIRES cells (control), and the cellular uptake of tested nucleotides was determined at various time points in the absence and presence of 500 μM probenecid (PBC). (B) hOAT3. Experiments were conducted under the same conditions except with longer incubation times and using BHKhOAT3 cells and BHKpIRES cells (control). The data for both transporters are presented as means ± standard deviations from two independent experiments performed in duplicate. At all analyzed time points, the accumulation of both drugs in the transporter-expressing cells was significantly different from that in the control cells and the transporter-expressing cells treated with PBC (P < 0.005 for hOAT1 and P < 0.01 for hOAT3 using a two-tailed paired t test).
hOAT1 and hOAT3 transport kinetics.
A more detailed assessment of transport kinetics demonstrated that GS-9148 has 5- to 10-fold lower affinity for hOAT1 compared to adefovir, tenofovir, and cidofovir (Table 1). The overall efficiency of hOAT1-mediated transport of GS-9148, determined as the Vmax/Km ratio, is 60- to 100-fold lower than that of the tested acyclic nucleotides. Although the affinity of GS-9148 toward hOAT3 was similar to that of adefovir and tenofovir, the overall efficiency of its transport by hOAT3 was three- to sixfold lower compared to the two acyclic nucleotides. Collectively, these data show that GS-9148 interacts with the studied renal uptake transporters substantially less efficiently than the acyclic nucleotides. The difference is especially obvious in the case of hOAT1.
TABLE 1.
Kinetics of hOAT1- and hOAT3-mediated transport of GS-9148 and acyclic nucleotide analogs
| Compound | hOAT1a
|
hOAT3a
|
||||
|---|---|---|---|---|---|---|
| Km (μM) | Vmax (pmol/min/106 cells) | Vmax/Km | Km(μM) | Vmax (pmol/min/106 cells) | Vmax/Km | |
| GS-9148 | 259 ± 102 | 7.8 ± 0.8 | 0.03 | 1,216 ± 8 | 19.5 ± 14.0 | 0.016 |
| Cidofovir | 58.0 ± 5.7 | 103 ± 10 | 1.77 | NDb | ND | ND |
| Adefovir | 23.8 ± 4.2 | 46.0 ± 4.4 | 1.93 | 1,220 ± 740 | 131.4 | 0.105 |
| Tenofovir | 33.8 ± 3.4 | 110 ± 12 | 3.26 | 770 ± 210 | 28.7 ± 8.6 | 0.037 |
Data represent mean ± standard deviations from two independent experiments performed in duplicate.
ND, not determined. Cidofovir has been shown not to be a substrate for hOAT3.
Cytotoxicity in hOAT1-expressing cells.
It has been demonstrated in prior studies that the expression of hOAT1 markedly enhances the cytotoxicity of cidofovir and adefovir due to a selective increase in their intracellular accumulation (15). In a side-by-side comparison with acyclic nucleotide analogs, GS-9148 showed 20- to 300-fold lower cytotoxicity in CHOhOAT1 cells (Table 2). In addition, the shift in GS-9148-induced cytotoxicity caused by functional overexpression of hOAT1 was approximately 5-fold, whereas cidofovir, adefovir, and tenofovir all exhibited 100- to 200-fold increases in their cytotoxicity upon the expression of the transporter. These results are consistent with the less effective intracellular accumulation of GS-9148 via the action of hOAT1.
TABLE 2.
hOAT1-mediated cytotoxicity of antiviral nucleotide analogs
| Compound | CC50 (μM)a
|
||
|---|---|---|---|
| CHOpIRES | CHOhOAT1 | Fold change | |
| Cidofovir | 520 ± 382 | 3.0 ± 1.4 | 173 |
| Adefovir | 255 ± 155 | 1.4 ± 0.7 | 182 |
| Tenofovir | ≥2,000 | 21.0 ± 7.0 | ≥95 |
| GS-9148 | ≥2,000 | 435 ± 148 | ≥4.5 |
Data represent mean ± standard deviation values from two independent experiments performed in triplicate.
Efflux by MRP4.
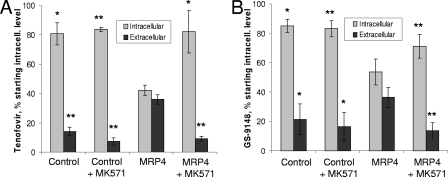
To determine whether GS-9148 is a substrate for MRP4 and thus can undergo elimination from proximal tubules, HEK-293T cells transiently expressing the efflux transporter were preloaded with radiolabeled prodrugs tenofovir disoproxil or GS-9131. It has been shown before that both prodrugs undergo effective intracellular conversion to the respective parent nucleotides (26, 27). The efflux of nucleotides from cells was followed in the presence and absence of the MRP4 inhibitor MK-571. Consistent with prior studies (4), MRP4 expression enhanced the elimination of tenofovir from cells, as evidenced by a lower intracellular concentration and a higher extracellular level of the compound following the incubation of preloaded transporter-expressing cells relative to controls (Fig. 3A). In addition, MK-571 increased the retention of tenofovir in MRP4-expressing cells but had no effect on the efflux of the compound from control cells. An identical profile was observed following the incubation of cells preloaded with GS-9131, indicating that GS-9148 is also actively effluxed by MRP4 (Fig. 3B). This conclusion was further confirmed by independent studies in CEM-R1 T cells, which are resistant to acyclic nucleotides such as adefovir due to drug-induced overexpression of MRP4 (29). CEM-R1 cells treated with 10 μM GS-9148 accumulated the compound and its phosphorylated metabolites to levels that were 3- to 10-fold lower than those in the control wild-type CEM-SS cells that lacked MRP4 (data not shown). Similar differences in intracellular drug accumulation between CEM-R1 and CEM-SS cells have previously been shown with tenofovir (25). Collectively, these data demonstrate that similar to tenofovir and adefovir, GS-9148 is also a substrate for MRP4 and thus can be actively eliminated from the MRP4-expressing cells and tissues, including renal proximal tubules.
FIG. 3.
MRP4-mediated efflux of tenofovir (A) and GS-9148 (B). Control cells and cells transiently expressing human MRP4 were preloaded with 1 μM [3H]tenofovir disoproxil (A) or 5 μM [14C]GS-9131 (B) for 2 h. After preloading, cells were washed and incubated in fresh medium in the presence or absence of 30 μM efflux inhibitor MK-571 for an additional 90 min. The amount of retained intracellular and effluxed extracellular compound was determined from the radioactivity content in cell lysates and culture medium, respectively, and is expressed as the percentage of the initial amount of compound present in preloaded cells. The data are presented as means ± standard deviations from three independent experiments performed in duplicate. The statistical significance of the differences between MRP4-expressing cells and the other tested samples for both intracellular and extracellular compound was assessed using a two-tail paired t test. *, P < 0.05; **, P < 0.01.
In vitro accumulation in human renal tissue.
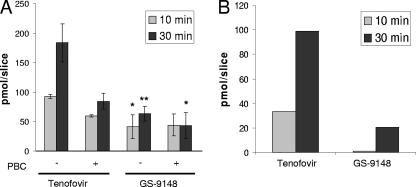
To understand the relative accumulation of GS-9148 and acyclic nucleotides, drug uptake studies were conducted using precision-cut tissue slices prepared from fresh human kidney cortex. At the concentration of 5 μM, the total accumulation of GS-9148 in renal tissue was two- to threefold lower than that of tenofovir (Fig. 4A). In addition, the uptake of tenofovir was substantially more sensitive to probenecid than the uptake of GS-9148, indicating a higher proportion of transporter-mediated accumulation of tenofovir, which likely corresponds to active transport of the drug into renal proximal tubules. As a result, the net probenecid-sensitive accumulation of GS-9148, which should represent primarily the drug present in proximal tubules, was approximately fivefold lower than that of tenofovir (Fig. 4B).
FIG. 4.
Accumulation of tenofovir and GS-9148 in fresh human kidney cortex slices. Precision-cut tissue slices were incubated with 5 μM [3H]tenofovir or [14C]GS-9148 in the presence or absence of 500 μM probenecid (PBC). Total (A) and probenecid-sensitive (B) accumulation of the tested nucleotides in tissue slices is shown. Data represent mean ± standard deviation values from a representative experiment performed in triplicate. The accumulation of GS-9148 in human renal tissue was significantly different from that of tenofovir under corresponding conditions. *, P < 0.05; **, P < 0.01.
In vivo renal distribution and urinary clearance of GS-9148 metabolites.
The effects of the molecular mechanism and kinetics of GS-9148 renal transport on kidney accumulation were measured following oral administration of 3 mg/kg [14C]GS-9131 to dogs. At 24 h postdosing, radiation was widely distributed, with the highest concentrations in liver, bile, kidney, gall bladder, and duodenum (data not shown). Levels in the kidney were 2.49 ± 0.81 μg equivalents/g of tissue, or 0.41 ± 0.12% of the administered dose (values are the means ± standard deviations for three dogs). In comparison, [14C]tenofovir disoproxil dosed orally to dogs at 10 mg/kg resulted in kidney levels of 89.9 ± 38.4 μg equivalents/g of tissue, representing 4.58% of the administered dose (n = 2 dogs) (20).
GS-9148 was the major species in urine, accounting for 84% of the material excreted in urine over 24 h. Based on the plasma exposure (area under the time-concentration curve from 0 to 24 h) of 4,900 nM·h measured for GS-9148 following administration of 3 mg/kg GS-9131 in a previously reported pharmacokinetic study (5), a clearance rate (CLr)for GS-9148 of approximately 0.47 liter/h/kg was calculated.
DISCUSSION
Nucleotide analogs carrying a negative charge under physiological conditions are substrates for the major renal organic anion transporters hOAT1 (3, 15), hOAT3 (31), and MRP4 (4, 16, 25). Results from prior in vitro and in vivo studies demonstrated that the efficient transport of antiviral acyclic nucleotides, primarily by hOAT1, mediates their selective renal accumulation that may, depending on intrinsic cytotoxicity and dose, lead to nephrotoxicity (3). The present study generated several lines of evidence indicating that GS-9148, compared to the characterized acyclic nucleotides, is less likely to accumulate in renal proximal tubule cells.
The interaction of GS-9148 with both hOAT1 and hOAT3 is less effective than cidofovir, adefovir, and tenofovir. Specifically, GS-9148 exhibits 5- to 10-fold lower affinity (Km) for hOAT1 and 60- to 100-fold lower overall transport efficiency (Vmax/Km) by the transporter compared to acyclic nucleotides. Although GS-9148 shows similar affinity for hOAT3 as adefovir and tenofovir, its transport efficiency is three- to fourfold lower than that of the acyclic nucleotides.
The net accumulation in proximal tubules of renally secreted small molecules is a result of a combined activity and equilibrium of tubular uptake and efflux. Our in vitro studies using two independent cell-based models established that GS-9148 is a substrate for MRP4, an important efflux pump present in proximal tubules, and the overexpression of MRP4 lowers the intracellular accumulation of GS-9148 and its metabolites. This indicates that MRP4 is likely involved in the active tubular secretion of GS-9148 and plays a role in controlling the intracellular accumulation of GS-9148 and its metabolites in proximal tubule cells.
Experiments conducted with fresh renal cortex slices, where the net combined effect of hOAT1, hOAT3, and MRP4 activity on the tissue accumulation could be assessed, showed approximately fivefold-lower levels of GS-9148 compared to tenofovir in tissue exposed to the two nucleotides under identical conditions. This observation is consistent with the relatively inefficient transport of GS-9148 by hOAT1 and hOAT3 and its ability to be effluxed by MRP4.
The potential for nephrotoxicity is defined by both the propensity to accumulate in proximal tubule cells and the intrinsic cytotoxicity of a given nucleotide. Although cidofovir, adefovir, and tenofovir show similar high efficiencies of transport by the major renal transporter hOAT1, they differ substantially in their in vitro cytotoxicities, and this appears to correlate with their different in vivo nephrotoxicity potentials (3). Here we showed that, consistent with its less effective transport by hOAT1 and low intrinsic cytotoxicity, GS-9148 is also markedly less cytotoxic in cells overexpressing hOAT1 than the comparative nucleotide analogs. The issue of intrinsic cytotoxicity of both GS-9148 and its prodrug GS-9131 has also been addressed in prior studies (5). Similar to tenofovir, GS-9148 at concentrations exceeding 1 mM exhibits minimal in vitro cytotoxicity in primary human renal proximal tubule cells from multiple donors as well as in other cell types (5). Inhibition of mitochondrial DNA polymerase γ by active metabolites of nucleoside analogs has been implicated in various clinical adverse effects (18, 33), and several authors suggested that it may also represent the underlying mechanism for the nucleotide-associated renal toxicity (6, 30). We have previously shown that the active diphosphate metabolite of GS-9148 is a poor inhibitor of mitochondrial DNA polymerase γ and neither GS-9148 nor its various prodrugs, including GS-9131, affect the levels of mitochondrial DNA in cells treated with supratherapeutic concentrations (5). Therefore, it is unlikely that the clinical administration of GS-9131 would result in mitochondrial toxicity in either kidneys or other organs or tissues.
In addition to data from in vitro renal transport and accumulation studies, tissue distribution and pharmacokinetic studies conducted with radiolabeled GS-9131 in dogs illustrate that GS-9148 accumulates to a lesser extent in kidneys in vivo and has a lower proportion of material cleared by active tubular secretion than acyclic nucleotide analogs. Dose-normalized concentrations of total material observed in the kidney tissue 24 h following the administration of [14C]GS-9131 were approximately sixfold lower than those previously reported following treatment with [14C]tenofovir disoproxil (20). The estimated CLr based on the amount of intact GS-9148 observed in the urine over 24 h was 0.47 liters/h/kg. This value is only slightly above the glomerular filtration rate (0.37 liters/h/kg) and markedly below renal blood flow (1.3 liters/h/kg) in the dog (12), suggesting only a limited contribution of the net active tubular secretion to the overall CLr of GS-9148. In fact, the predominant mode of GS-9148 renal elimination in the dog is likely passive filtration at the glomerulus. This is in contrast to acyclic nucleotide analogs, for which CLr has been measured to be in excess of twofold above the glomerular filtration rate in various species, including human, (1, 7, 8, 11). The reduced renal accumulation and elimination by active tubular secretion of GS-9148 are consistent with the lack of renal findings in Sprague-Dawley rats, beagle dogs, and cynomolgus monkeys treated orally with GS-9131 for 28 days at doses up to 300, 20, and 30 mg/kg/day, respectively.
Prior in vitro and in vivo pharmacokinetic profiling of GS-9131 and its metabolites in blood and PBMCs provided a basis for the estimation of clinical exposure that could lead to a therapeutic effect in treated patients (5, 26). Following the oral administration of GS-9131 to dogs at 3 mg/kg, plasma exposure of GS-9148 was comparable to that of tenofovir in patients treated with a standard 300-mg clinical dose of tenofovir disoproxil fumarate, whereas the concentration of GS-9148 diphosphate in PBMCs from treated dogs approached levels approximately 20-fold higher compared to intracellular concentrations of tenofovir diphosphate detected in the clinic (5, 14). Considering the similar intracellular antiviral potency of GS-9148 diphosphate and tenofovir diphosphate in HIV-infected PBMCs in vitro (26), these observations suggest that the plasma levels of GS-9148 at therapeutically active doses of GS-9131 should be well below the clinical plasma levels of tenofovir. This should further reduce the renal exposure to GS-9148 compared to acyclic nucleotides.
In conclusion, we have established that, similar to acyclic nucleotide analogs, GS-9148 is a substrate for renal transporters hOAT1, hOAT3, and MRP4. However, the specific kinetics of these interactions result in reduced levels of active renal accumulation of GS-9148 relative to acyclic nucleotides, a difference observed both in the in vitro and in vivo models. Limited renal accumulation, coupled with low intrinsic cytotoxicity and expected reduced plasma exposure, suggests a low potential of GS-9148 to cause renal tubular dysfunction in patients treated with its prodrug, GS-9131.
Acknowledgments
We thank Hans Reiser and Mick Hitchock of Gilead Sciences for reviewing the manuscript and providing insightful comments.
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boojamra, C. G., R. L. Mackman, D. Y. Markevitch, V. Prasad, A. S. Ray, J. Douglas, D. Grant, C. U. Kim, and T. Cihlar. 2008. Synthesis and anti-HIV activity of GS-9148 (2′-Fd4AP), a novel nucleoside phosphonate HIV reverse transcriptase inhibitor. Bioorg Med. Chem. Lett. 18:1120-1123. [DOI] [PubMed] [Google Scholar]
- 3.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 4.Cihlar, T., A. Ray, G. Laflamme, J. Vella, L. Tong, M. Fuller, A. Roy, and G. Rhodes. 2007. Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors. Antivir. Ther. 12:267-272. [PubMed] [Google Scholar]
- 5.Cihlar, T., A. S. Ray, C. G. Boojamra, L. Zhang, H. Hui, G. Laflamme, J. E. Vela, D. Grant, J. Chen, F. Myrick, K. L. White, Y. Gao, K. Y. Lin, J. L. Douglas, N. T. Parkin, A. Carey, R. Pakdaman, and R. L. Mackman. 2008. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 52:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cote, H. C., A. B. Magil, M. Harris, B. J. Scarth, I. Gadawski, N. Wang, E. Yu, B. Yip, N. Zalunardo, R. Werb, R. Hogg, P. R. Harrigan, and J. S. Montaner. 2006. Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antivir. Ther. 11:79-86. [PubMed] [Google Scholar]
- 7.Cundy, K. C. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 8.Cundy, K. C., P. Barditch-Crovo, R. E. Walker, A. C. Collier, D. Ebeling, J. Toole, and H. S. Jaffe. 1995. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob. Agents Chemother. 39:2401-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cundy, K. C., A. M. Bidgood, G. Lynch, J. P. Shaw, L. Griffin, and W. A. Lee. 1996. Pharmacokinetics, bioavailability, metabolism, and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab. Dispos. 24:745-752. [PubMed] [Google Scholar]
- 10.Cundy, K. C., Z. H. Li, and W. A. Lee. 1996. Effect of probenecid on the distribution, metabolism, and excretion of cidofovir in rabbits. Drug Metab. Dispos. 24:315-321. [PubMed] [Google Scholar]
- 11.Cundy, K. C., B. G. Petty, J. Flaherty, P. E. Fisher, M. A. Polis, M. Wachsman, P. S. Lietman, J. P. Lalezari, M. J. M. Hitchcock, and H. S. Jaffe. 1995. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 39:1247-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 13.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T.-T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, J. Ma, S. Arterburn, S. Xiong, G. Currie, and C. L. Brosgart. 2005. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 352:2673-2681. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins, T., W. Veikley, R. L. I. St. Claire, B. Guyer, N. Clark, and B. P. Kearney. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406-411. [DOI] [PubMed] [Google Scholar]
- 15.Ho, E. S., D. C. Lin, D. B. Mendel, and T. Cihlar. 2000. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J. Am. Soc. Nephrol. 11:383-393. [DOI] [PubMed] [Google Scholar]
- 16.Imaoka, T., H. Kusuhara, M. Adachi, J. D. Schuetz, K. Takeuchi, and Y. Sugiyama. 2007. Functional involvement of multidrug resistance associated protein 4 (MRP4/ABCC4) in the renal elimination of the anti-viral drugs, adefovir and tenofovir. Mol. Pharmacol. 71:619-627. [DOI] [PubMed] [Google Scholar]
- 17.Izzedine, H., V. Launay-Vacher, and G. Deray. 2005. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 45:804-817. [DOI] [PubMed] [Google Scholar]
- 18.Kakuda, T. N. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochrondrial toxicity. Clin. Ther. 22:685-708. [DOI] [PubMed] [Google Scholar]
- 19.Lalezari, J. P., R. J. Stagg, B. D. Kuppermann, G. N. Holland, F. Kramer, D. V. Ives, M. Youle, M. R. Robinson, W. L. Drew, and H. S. Jaffe. 1997. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann. Intern. Med. 126:257-263. [DOI] [PubMed] [Google Scholar]
- 20.Lee, W. A., G.-X. He, E. Eisenberg, T. Cihlar, S. Swaminathan, A. Mulato, and K. C. Cundy. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49:1898-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyseng-Williamson, K., N. Reynolds, and G. Plosker. 2005. Tenofovir disoproxil fumarate: a review of its use in the management of HIV infection. Drugs 65:413-432. [DOI] [PubMed] [Google Scholar]
- 22.Motohashi, H., Y. Sakurai, H. Saito, S. Masuda, Y. Urakami, M. Goto, A. Fukatsu, O. Ogawa, and K.-I. Inui. 2002. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 13:866-874. [DOI] [PubMed] [Google Scholar]
- 23.Perazella, M. A. 2003. Drug-induced renal failure: update on new medications and unique mechanisms of nephrotoxicity. Am. J. Med. Sci. 325:349-362. [DOI] [PubMed] [Google Scholar]
- 24.Pham, P. A., and J. E. Gallant. 2006. Tenofovir disoproxil fumarate for the treatment of HIV infection. Expert Opin. Drug Metab. Toxicol. 2:459-469. [DOI] [PubMed] [Google Scholar]
- 25.Ray, A. S., T. Cihlar, K. L. Robinson, L. Tong, J. E. Vela, M. D. Fuller, L. M. Wieman, E. J. Eisenberg, and G. R. Rhodes. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray, A. S., J. E. Vela, C. G. Boojamra, L. Zhang, H. Hui, C. Callebaut, K. Stray, K. Y. Lin, Y. Gao, R. L. Mackman, and T. Cihlar. 2008. Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent. Antimicrob. Agents Chemother. 52:648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins, B. L., R. V. Srinivas, C. Kim, N. Bischofberger, and A. Fridland. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl) PMPA. Antimicrob. Agents Chemother. 42:612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sax, P. E., J. E. Gallant, and P. E. Klotman. 2007. Renal safety of tenofovir disoproxil fumarate. AIDS Read. 17(C3):90-92, 99-104. [PubMed] [Google Scholar]
- 29.Schuetz, J. D., M. C. Connelly, D. Sun, S. G. Paibir, P. M. Flynn, R. V. Srivinas, A. Kumar, and A. Fridland. 1999. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat. Med. 5:1048-1051. [DOI] [PubMed] [Google Scholar]
- 30.Tanji, N., K. Tanji, N. Kambham, G. S. Markowitz, A. Bell, and V. D. D'Agati. 2001. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum. Pathol. 32:734-740. [DOI] [PubMed] [Google Scholar]
- 31.Uwai, Y., H. Ida, Y. Tsuji, T. Katsura, and K. Inui. 2007. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm. Res. 24:811-815. [DOI] [PubMed] [Google Scholar]
- 32.van Aubel, R. A., P. H. E. Smeets, J. G. P. Peters, R. J. M. Bindels, and F. G. M. Russel. 2002. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J. Am. Soc. Nephrol. 13:595-603. [DOI] [PubMed] [Google Scholar]
- 33.White, A. J. 2001. Mitochondrial toxicity and HIV therapy. Sex. Transm. Infect. 77:158-173. [DOI] [PMC free article] [PubMed] [Google Scholar]