Abstract
High chloroquine doses are commonly prescribed in Guinea-Bissau. Double-dose chloroquine has been shown to be more efficacious (92% efficacy) than the standard dose (80% efficacy). However, chloroquine is toxic when overdosed, and it was not known if the high doses prescribed in Guinea-Bissau were taken or whether they caused adverse effects. We aimed to determine the dosage of chloroquine commonly prescribed, the doses commonly taken, and whether concentration-dependent adverse events occurred in routine practice. Chloroquine prescriptions by eight physicians and chloroquine intake by 102 children were recorded. Chloroquine intake and adverse events were assessed by questioning. Chloroquine concentrations in whole blood were measured. The median total chloroquine dose prescribed and that reportedly taken were 81 and 77 mg kg−1, respectively. The total dose was usually split into two to three daily doses of 6.6 mg kg−1 each. These were taken unsupervised for a median of 5 days. Forty percent of the study children had chloroquine concentrations in the same range as those found in a previous study in which double the normal dose (50 mg kg−1) of chloroquine was taken. Only 3/102 children had Plasmodium falciparum in the blood at the time of diagnosis and treatment. No severe adverse events were reported. No adverse events were associated with higher chloroquine concentrations. High doses of chloroquine are commonly taken and well tolerated in Guinea-Bissau. Malaria diagnostics are poor, and chloroquine is commonly prescribed to children without parasitemia. Use of high-dose chloroquine is concurrent with an exceptionally low prevalence of chloroquine-resistant P. falciparum.
Chloroquine-resistant (CQR) Plasmodium falciparum spread through Africa during the '80s and '90s and was first described in Guinea-Bissau in 1990 (5). Until June 2008, chloroquine (CQ) remained by far the most commonly used antimalarial in the country. In Guinea-Bissau, as in most other areas of Africa, CQR is associated with a mutation in the CQR transporter (pfcrt K76T) (22, 25). Despite the presence of the pfcrt K76T mutation and continued CQ use, which should select CQR P. falciparum, the prevalence of CQR P. falciparum is exceptionally low and unchanged in Guinea-Bissau (23).
In Guinea-Bissau, treatment with the standard total CQ dose of 25 mg kg−1 of body weight given over 3 days had an efficacy of 80% (8). Treatment with 50 mg kg−1 of CQ in two divided daily doses over 3 days resulted in a 92% PCR-corrected efficacy at day 28 (8). When 50 mg kg−1 was used, 78% of infections with P. falciparum carrying the CQR-associated genetic marker (pfcrt 76T) were successfully treated, while only 34% were successfully treated with 25 mg kg−1 (22). The WHO recommendation that an antimalarial drug should have a minimum 90% efficacy was therefore still attained with CQ in Guinea-Bissau (24).
CQ is rapidly and almost completely absorbed. The peak concentration is obtained 1 to 3 h after oral intake, and 50 to 65% is protein bound in plasma. The concentration in whole blood is approximately 10 times higher than the concentration in plasma after intake of a single oral dose (4). Following absorption, CQ is distributed throughout the body, accumulating in tissues, especially the liver, lungs, spleen, and kidneys. The volume of distribution of CQ is very large (>100 liters/kg) (9). When overdosed, CQ is highly toxic, causing death soon after ingestion (13, 20). CQ is metabolized to desethylchloroquine (DCQ), which has a longer half-life than CQ.
We have previously reported that high CQ doses are commonly prescribed in one health center in Guinea Bissau (23) and that higher doses are more efficacious (6-8). The primary objectives of this observational study were therefore to monitor CQ prescription practices by clinicians and reported CQ consumption by children. The secondary objectives were to collect information on adverse events and CQ concentrations in order to determine if higher CQ doses were commonly used and whether they were well tolerated or not. Ultimately the aim was to improve our understanding of why CQR remains uncommon in Guinea-Bissau.
MATERIALS AND METHODS
Location.
The study was conducted at the Bandim Health Project in Bissau, Guinea-Bissau (population, approximately 92,000). The Bandim Health Project is a Demographic Survey Site (DSS) that comprises mainly semiurban areas. As part of the health services provided to the community, CQ has been available free of charge from a drug dispensary.
Malaria epidemiology and seasonality.
Children develop symptomatic P. falciparum malaria infections all year round in Guinea-Bissau. However, there is seasonality, with higher transmission rates between May and December just before, during, and after the rainy season that lasts from June to October/November. Monitoring of slide-positive malaria in 2003 to 2004 among all children seeking medical help at the three outpatient clinics serving the study area found 751/12,927 (5.8%) and 1,162/13,207 (8.8%) cases during the dry and wet seasons, respectively, in children under the age of 5 years (16). The malaria incidence has decreased over the years. In 1990, 183/312 (59%) children 3 to 6 years old had P. falciparum during the rainy season according to community surveys, while in 2004, the incidence was 7/197 (3.6%) children <5 years old (16).
Recruitment.
The study was conducted between January and March 2006. Children presenting at the drug dispensary with a diagnosis of malaria from a physician and a CQ prescription were eligible for participation. The dry season was chosen so as to prevent our being overwhelmed by patients and therefore unable to follow them.
Follow-up.
Table 1 shows the follow-up schedule. Upon inclusion, prescriptions were copied, and the mother or legal guardian was asked about drug intake and symptoms prior to study entry. Finger prick blood was drawn for thick and thin films and for CQ analysis.
TABLE 1.
Follow-up schedule
| Action | Performed or not performeda at the following time:
|
||||
|---|---|---|---|---|---|
| Day 0 | Each treatment day until the end of treatment | End of treatment + 1 day | End of treatment + 4 days | End of treatment + 14 days | |
| Questionnaire | + | + | + | + | + |
| Thick and thin smears | + | + | + | ||
| Collection of 100 μl of blood for CQ analysis | + | + | + | + | |
+, the action was taken. A blank cell indicates that a particular action was not taken.
The child was then visited once daily in the afternoon. During the visit, the mother was asked about drug intake and the symptoms that her child had experienced since the previous visit. When the mother reported that she was no longer giving CQ, a new blood sample was taken for CQ analysis and microscopy. Data and blood were thus collected 1 day after the last dose. The child was then visited two additional times, 4 and 14 days after the last dose. At each visit, the mother or guardian was questioned about symptoms and drug intake. Blood was drawn for CQ analysis and (on the last visit) also for microscopy. Children who were not seen for two consecutive visits were excluded.
After the end of the study, Projecto de Saùde de Bandim health records were searched to identify any children who had died during the study period.
Questions asked.
At each visit, the mother was asked what drugs as well as what doses and how many such doses the child had been given. The mother was also asked if the child had appeared to suffer from any of the following symptoms: fever, nausea, vomiting, diarrhea, stomach pain, cough, rapid breathing, itch, pallor, paresthesias, or convulsions. In addition, open questions were asked about the child's current health status.
Microscopy.
Thick and thin smears were made from capillary blood, and slides were stained with Giemsa stain. Because this was an observational study, the slides were not examined until after the end of the study. Slides were then examined by two experienced lab technicians. Both examined all the slides and counted parasites per 200 leukocytes.
CQ concentrations.
Exactly 100 μl of finger prick blood was collected using a capillary tube. The blood was put on filter paper (3M; Whatman, Brentford, United Kingdom) and dried. Each filter paper was then placed in a separate plastic bag and stored for approximately 6 months prior to analysis. Great care was taken to ensure that the filter papers were not contaminated with CQ from other sources. Concentrations of CQ and its metabolite DCQ were assessed by high-performance liquid chromatography as previously described (11).
Ethical approval.
Ethical approval was granted by the ethical review board in Guinea-Bissau (Parecer NCP/N 19/2006).
Statistics.
The data were entered by an assistant, and 50% of the data entered was double-checked by one of the authors. Statistical analysis used the nonparametric Kruskal-Wallis test, the nonparametric test for trend, and logistic regression analyses. To detect concentration-dependent side effects, the data were divided into groups above and below the mean and median values and into quartiles according to CQ concentrations 1 and 4 days after end of therapy.
RESULTS
Patients.
Following informed consent, 114 children were included. Five were subsequently excluded because the exact amount of CQ prescribed was unclear. One was excluded because of residence outside of Bandim. Six were excluded because they were not seen on two consecutive visits. The analysis is therefore based on 102 children, of whom 50 were boys and 52 girls. The median age was 14.7 months (range, 6 to 56 months). The 113 children residing within the DSS were alive 3 to 6 months after study entry according to DSS records. The health of the child residing outside Bandim is unknown.
Malaria incidence.
Only 3 of the 102 children were later found to have P. falciparum in their blood by examination of the thick and thin smears made on the day of inclusion. The levels of parasitemia were 1,920, 160, and 120 parasites per μl of blood, assuming a white blood cell count of 8,000/μl. No parasites were identified during the follow-up. All children reported suffering from fever prior to entry. The median duration of fever was 2 days (interquartile range, 2 to 3 days; range, 1 to 14 days).
Prescriptions and reported consumption.
Eight different clinicians prescribed medications. All children were prescribed CQ. A quinine injection was prescribed to one child at the start of treatment. No other antimalarials were prescribed. Paracetamol was prescribed to 98 of the 102 children.
Fifty-one children were prescribed CQ syrup, and 51 were prescribed tablets. The children prescribed syrup were younger (9 versus 24 months; P < 0.001) and weighed less (8 versus 11 kg; P < 0.001) than those prescribed tablets. Sex was not associated with prescription patterns or the reported intake of CQ.
For children prescribed CQ tablets, the amount of CQ in each dose and the total amount of CQ prescribed were greater, while the number of doses per day was fewer, than those for children receiving syrup (Table 2). The reported amount of CQ taken in each dose was also larger for children taking tablets. CQ syrup and tablets were prescribed for the same time, but children taking tablets stopped earlier.
TABLE 2.
CQ prescriptions and reported CQ consumption
| Form | Consumption (median [interquartile range])a
|
|||
|---|---|---|---|---|
| Amt per dose (mg kg−1) | No. of daily doses | No. of days | Total amt (mg kg−1) | |
| CQ syrup | ||||
| Prescribed | 6.1 (5.5-7.2)b | 3 (2-3)c | 5 (5-5) | 76 (58-88)h |
| Reportedly taken | 6.1 (5.5-7.2)d | 3 (2-3) | 5 (4-5)e | 77 (53-99) |
| CQ tablets | ||||
| Prescribed | 8.9 (5.8-10.1)b | 2 (2-3)c,g | 5 (5-5) | 87 (77-99)h |
| Reportedly taken | 9 (5.9-10.2)d | 3 (2-3)g | 4 (3-5)e | 75 (53-114) |
| All | ||||
| Prescribed | 6.5 (5.7-9.5) | 2 (2-3)f | 5 (5-5) | 81 (72-92) |
| Reportedly taken | 6.6 (5.7-9.5) | 3 (2-3)f | 5 (4-5) | 77 (53-102) |
Values that were compared and found to be significantly different have the same footnote letter and the P value given is that found using the Kruskal-Wallis test.
P = 0.0003.
P = 0.05.
P = 0.0002.
P = 0.05.
P = 0.01.
P = 0.003.
P = 0.003.
CQ and DCQ concentrations in blood.
The CQ and DCQ concentrations in blood are shown in Table 3, Fig. 1, and Fig. 2. The child with the highest CQ concentration on day 0 (4,890 nM) had a DCQ/CQ proportion of 22%. He had diarrhea and a cough and had taken three doses of CQ each day during the 4 days prior to study entry. Reportedly, he continued taking CQ for 5 days, and his condition improved. One day and 14 days after intake of the last dose, his CQ concentrations were 2,830 nM and 837 nM, respectively. He was not available for sampling at 4 days.
TABLE 3.
Median CQ and DCQ concentrations in whole blood
| Day | Concn (nM)a
|
DCQ/CQ (%)a,b | No. of samples analyzed | |
|---|---|---|---|---|
| CQ | DCQ | |||
| 0 | 0 (0-205) | 0 (0-135) | 56 (34-86) | 96 |
| 1 | 894 (383-1,845) | 638 (284-1,035) | 64 (47-76) | 97 |
| 4 | 626 (290-1,630) | 446 (225-822) | 70 (51-88) | 85 |
| 14 | 204 (104-365) | 181 (81-292) | 81 (63-99) | 82 |
Given as median (interquartile range).
DCQ was divided by CQ for each sample, and then the median proportion was calculated.
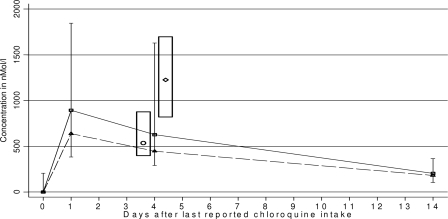
FIG. 1.
Median CQ and DCQ concentrations in whole blood. □, median CQ concentration with the interquartile range; ▵, median DCQ concentration; ○ and ⋄, median CQ concentration after observed intake of 25 or 50 mg kg−1, respectively (interquartile range within the column). The median CQ concentrations 4 days after completion of a 3-day treatment with 25 mg kg−1 (○) and 50 mg kg−1 (⋄) were 534 nM (interquartile range, 395 to 876 nM) and 1,225 nM (interquartile range, 820 to 1,698 nM), respectively (4). In this study, 4 days after the end of treatment, 34/85 children (40%) had CQ concentrations above 820 nM, 25/85 (29%) had CQ concentrations between 395 and 876 nM, 57/85 (67%) had CQ concentrations above 395 nM, and 28/85 (33%) had CQ concentrations below 395 nM.
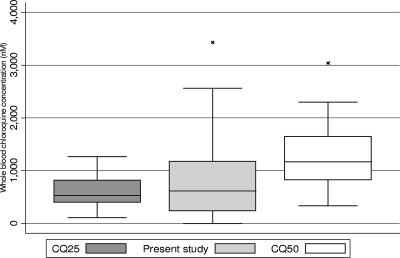
FIG. 2.
CQ concentrations in whole blood measured in this study compared with those found after observed intake of 25 or 50 mg kg−1. Three outliers are not shown (CQ25, 5,490 nM; CQ at day 4, 5,440 nM; CQ50, 4,780 nM). The line in each box represents the median CQ concentration. The box represents the interquartile range, and the range spike represents the 5th to 95th percentiles. CQ25 and CQ50 are the CQ concentrations determined 4 days after observed intake of 25 mg kg−1 and 50 mg kg−1 split into one and two daily doses, respectively, over 3 days. CQ concentrations shown for the present study were measured 4 days after the last reported dose. For the median and interquartile-range values, see the legend to Fig. 1.
Only 16 children reported intake of CQ during the week preceding study entry, but 39/96 (41%) had CQ and 44/96 (46%) had DCQ in the blood at the start of the study, indicating earlier intake. DCQ has a longer half-life than CQ, but concentrations of CQ are initially higher than those of DCQ. The difference between DCQ and CQ concentrations therefore decreases with time after intake unless more CQ is taken. In light of this, we noted that 69% of children (27/39) had DCQ/CQ proportions of 80% or less and 31% (12/39) had proportions between 80% and 171%. There was no correlation between the CQ concentration and the DCQ/CQ proportion. However, the presence of CQ in the blood prior to the start of treatment was associated with higher CQ concentrations 1 (1,590 versus 678 nM), 4 (991 versus 459 nM), and 14 (287 versus 133 nM) days after the end of treatment (P < 0.001).
CQ and DCQ concentrations did not vary with age or sex, with one exception. Four days after the end of treatment, male sex was associated with lower CQ concentrations (649 versus 1,207 nM; P = 0.004).
One day after the end of treatment, median CQ concentrations in blood were 632, 1,232, and 1,415 nM when one, two, and three daily doses were prescribed, respectively. The difference was not significant by nonparametric tests, probably because only five children were prescribed one daily dose.
Three patients had higher CQ concentrations on day 14 than they did on day 4, even though they did not admit to taking CQ between these days. The day 4 and 14 values were 1,960 and 3,190 nM, 1,790 and 5,120 nM, and 2,180 and 5,437 nM, respectively. DCQ was present in all three patients in proportions closer to those found on day 1 than to those commonly found on day 14 (44%, 42%, and 44%), in line with additional intake (Table 3).
The concentrations obtained 4 days after the last dose in this study were compared to concentrations found 4 days after supervised intake of normal- and double-dose CQ (25 and 50 mg kg−1 in 3 days, respectively) in a previous study (Fig. 1 and 2). Because CQ concentrations differ considerably even after supervised intake, the values obtained in the previous study were split into centiles. Forty percent (34/85) of children in the present study had CQ concentrations above the 25th percentile and 68% (58/85) had CQ concentrations above the 5th percentile found after supervised intake of 50 mg kg−1 in the previous study (8).
Median DCQ/CQ proportions and interquartile ranges 1, 4, and 14 days after the end of treatment were 0.64 (0.46 to 0.76), 0.69 (0.51 to 0.89), and 0.81(0.62 to 1.0), respectively. The difference between DCQ and CQ concentrations decreased significantly (P < 0.001 by the nonparametric test for trend).
Adverse events.
No reported symptoms were associated with higher CQ concentrations (Table 4). Diarrhea and vomiting prior to study entry were associated with lower CQ concentrations (P = 0.02 and P = 0.006, respectively, by the nonparametric test for trend). Diarrhea on the first day of follow-up was also associated with lower CQ concentrations (P = 0.009). Rapid breathing prior to study entry was associated with lower CQ concentrations (P = 0.006).
TABLE 4.
Symptoms and their association with CQ concentrations
| Quartilea | CQ concn (nmol/liter)b 1 day after end of treatment | Frequencyc of the following symptom:
|
Duration of vomiting (days)d | |||
|---|---|---|---|---|---|---|
| Vomiting prior to study entry | Vomiting on day 1 | Diarrhea prior to study entry | Diarrhea on day 1 | |||
| 1 | 185 (0-370) | 13/24 (54) | 6/24 (25) | 13/24 (54) | 10/24 (42) | 1.4 (0.8-2.0) |
| 2 | 662 (395-894) | 14/25 (56) | 6/25 (24) | 15/25 (60) | 16/25 (64) | 1.1 (0.7-1.5) |
| 3 | 1,412 (961-1,820) | 4/24 (17) | 3/21 (14)e | 9/24 (38) | 7/21 (33)e | 0.5 (0-1) |
| 4 | 3,035 (1,870-5,380) | 6/24 (25) | 2/24 (8) | 7/24 (29) | 5/24 (21) | 0.5 (0.2-0.9) |
| Undefined | 2/5 (40%) | 0/5 (0) | 2/3 (40)f | 0/5 (0) | 0.4 (0-1) | |
Defined on the basis of CQ concentrations. The “undefined” group comprised those for whom blood was not available for CQ concentration analysis 1 day after the end of treatment.
The range is given in parentheses.
Given as the number of patients with the symptom/total number of patients (percentage). Using the nonparametric test for trend vomiting prior to study entry (P = 0.006), vomiting on day 1 (P = 0.009), diarrhea prior to study entry (P = 0.03), and diarrhea on day 1 (P = 0.04) were more common in children with lower CQ concentrations.
The 95% confidence interval is given in parentheses. The P value as assessed by the nonparametric test for trend is 0.002.
Data were not available for three children.
Data were not available for two children.
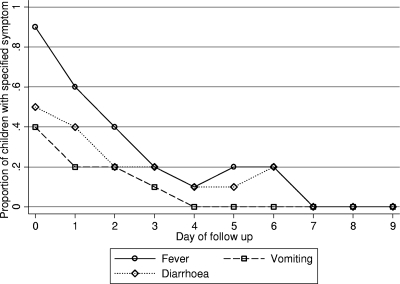
Lower CQ concentrations were associated with vomiting for a longer period. The mean durations of vomiting were 1.4, 1.1, 0.5, and 0.5 days in the quartiles with the lowest to highest CQ concentrations, respectively (P = 0.002 by a nonparametric test for trend). No other association between the duration of a symptom and the CQ concentration was found. The mean symptom durations (Fig. 3), in days (with 95% confidence intervals in parentheses), were as follows: fever, 2.3 (2.1 to 2.6); vomiting, 0.9 (0.6 to 1.1); diarrhea, 1.5 (1.2 to 1.8); cough, 1.9 (1.6 to 2.3); nausea, 0.3 (0.1 to 0.5); pallor, 0.3 (0.07 to 0.4); stomach pain, 0.2 (0.08 to 0.3). Other symptoms were infrequent and were not associated with CQ concentrations.
FIG. 3.
Duration of fever, vomiting, and diarrhea. These symptoms were associated with lower CQ concentrations.
DISCUSSION
In routine practice, approximately three times the WHO-recommended dose of CQ (25 mg kg−1) was prescribed and reportedly taken as split daily doses over 5 days. Measurement of CQ concentrations in blood indicated that at least 40% of the children attained concentrations that were the same as, or greater than, those measured after supervised intake of double-dose (50-mg kg−1) CQ. The data confirm that high doses were used and indicate that they were well tolerated in Bissau.
There is a discrepancy between reported intake and determined CQ concentrations. This might be partly explained by young children spitting and spluttering, and thereby not swallowing the whole dose given. It is also possible that mothers reported giving CQ when they did not. Consequently, some CQ concentrations may have been measured, not 1 day after the last dose, but later, resulting in an underestimation of the proportion of children with CQ concentrations similar to those found after patients had taken 50 mg kg−1.
CQ's toxicity is well documented (13, 20), yet we observed no concentration-dependent adverse events or severe adverse events. Peak concentrations occur 1 to 6 h after intake of CQ, and severe adverse events usually occur soon after intake of a high dose, suggesting that toxicity is caused by high peak concentrations or a rapid increase in the CQ concentration (3, 4, 9, 12, 15). The lack of severe adverse events in this study is probably explained by the use of repeated small doses (6.5 mg kg−1 dose−1). Small doses will cause smaller peak concentrations, and the repeated dosing allows time for distribution between doses so that toxic concentrations do not accumulate in the blood. Because CQ's volume of distribution is so large, a large total amount of CQ can probably be taken in this way before toxic concentrations are reached. These results are in line with our previous controlled studies (6-8), in which 50 mg kg−1 of CQ taken as split daily doses was well tolerated. Similarly, a loading dose of 20 mg kg−1 given as split doses during the first 24 h was well tolerated (15).
Though we did not detect any serious adverse events, our results do not exclude that possibility. In particular, CQ is known to be cardiotoxic (12), and monitoring of the blood pressure and pulse, as well as electrocardiograms, would have been of value. The association of diarrhea, vomiting, and rapid breathing with lower CQ concentrations prior to study entry and on the first day of the study was probably due to an epidemic of viral gastroenteritis. Children with gastroenteritis are probably less likely to take CQ and are likely to have impaired absorption due to vomiting, resulting in the lower CQ concentrations observed in this group. Furthermore, viral gastroenteritis commonly resolves spontaneously after a few days, in line with our results (Fig. 3).
The difference between DCQ and CQ concentrations decreased after the end of treatment (P < 0.001). This is in line with the longer half-life of DCQ (4) and indicates that additional CQ was rarely used during the observation period.
CQ was frequently prescribed to children who had recently taken CQ. This should result in higher CQ concentrations and, possibly, more adverse effects. As expected, children with CQ in the blood at study entry had higher CQ concentrations during the follow-up, but they did not suffer more-frequent adverse events. The repetitive use of small doses might explain the lack of adverse events, as discussed above. Poor diagnostics and overprescription of CQ are potential problems irrespective of whether a normal CQ dose is used or not.
Only 3/102 children had parasites in the blood, but all children had reportedly had fever prior to study entry, suggesting that antimalarial treatment is given routinely if there is a history of fever. Our experience is that microscopy is usually not performed, both in order to save time and minimize cost and because a negative result will not preclude treatment. The poor diagnostics or poor use of test results is not unique to the eight physicians in this study. In Bandim in 2003 to 2004, P. falciparum was identified only in 11% (751/6,941) and 14% (1,162/8,066) of children under the age of 5 years treated for malaria during the dry and rainy seasons, respectively (16). Similarly, only 2% (153/7,415) of children treated for malaria had >20 P. falciparum parasites per 200 white blood cells in 2007 (our unpublished data). In the latter two studies, clinicians were aware of the negative microscopy results but still prescribed CQ. Essentially, the chances of being treated for malaria are considerable each time a child visits a health center, irrespective of whether he or she has symptomatic malaria or not. Because microscopy often is not done, many asymptomatic parasitemias are probably treated, indicating that the approximately 12,000 children under the age of 5 living in the Bandim DSS are receiving some form of intermittent preventive therapy. Though intermittent preventive therapy with CQ may enhance the acquisition of immunity similarly to intermittent preventive therapy with sulfadoxine-pyrimethamine (18, 19, 21), overuse of CQ is expected to result in a rapid expansion of CQR P. falciparum. Yet CQ remains efficacious and pfcrt(K76T) prevalence stable, around 25%, in Guinea-Bissau (8, 23).
A probable explanation for the low 76T prevalence is the use of more-efficacious high-dose CQ combined with the loss of fitness associated with CQR. The reemergence of the CQ-sensitive 76K genotype in Malawi following the removal of CQ (10) and the seasonal variation of 76K/76T genotypes in Sudan (1) and The Gambia (14) suggest that the 76K genotype is inherently fitter than the 76T genotype. CQR is achieved by energy-dependent removal of CQ from the parasite's food vacuole (2, 17). When higher doses are used for longer periods, removal of CQ will require even more energy, at a further cost to parasite fitness. The lack of commonly occurring P. falciparum parasites resistant to high-dose CQ suggests that the 76T-associated resistance mechanism does not provide enough benefit for the genotype to become dominant in Guinea-Bissau, where high-dose CQ is commonly taken. Yet the 76T P. falciparum has an advantage when CQ concentrations are moderately high, such as soon after treatment. It is probable that a balance between resistant (76T) and sensitive (76K) P. falciparum, dependent on the amount of CQ used, inherent parasite fitness, and transmission intensity, is thus established.
In summary, we show that high doses of CQ are commonly taken and well tolerated in Guinea-Bissau and that malaria is exceedingly overdiagnosed. It is probable that adverse events are avoided by the use of repeated small doses. We note that the high-dose CQ treatment schedule used is concurrent with the continued efficacy of CQ and that both are unique to Guinea-Bissau. We believe that the situation in Guinea-Bissau warrants further research into dosing strategies, adverse events, and the combination possibilities of a drug that is cheap and available and to which efficacy returns when it is removed.
Acknowledgments
The Center for Clinical Research in Sörmlands läns landsting in Sweden partially funded this study.
We thank Peter Aaby for valuable comments.
None of the authors have any conflict of interest.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Abdel-Muhsin, A. M., M. J. Mackinnon, E. Ali, E.-K. A. Nassir, S. Suleiman, S. Ahmed, D. Walliker, and H. A. Babiker. 2004. Evolution of drug-resistance genes in Plasmodium falciparum in an area of seasonal malaria transmission in Eastern Sudan. J. Infect. Dis. 189:1239-1244. [DOI] [PubMed] [Google Scholar]
- 2.Bray, P. G., M. Mungthin, I. M. Hastings, G. A. Biagini, D. K. Saidu, V. Lakshmanan, D. J. Johnson, R. H. Hughes, P. A. Stocks, P. M. O'Neill, D. A. Fidock, D. C. Warhurst, and S. A. Ward. 2006. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Mol. Microbiol. 62:238-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemessy, J. L., P. Taboulet, J. R. Hoffman, P. Hantson, P. Barriot, C. Bismuth, and F. J. Baud. 1996. Treatment of acute chloroquine poisoning: a 5-year experience. Crit. Care Med. 24:1189-1195. [DOI] [PubMed] [Google Scholar]
- 4.Frisk-Holmberg, M., Y. Bergqvist, E. Termond, and B. Domeij-Nyberg. 1984. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur. J. Clin. Pharmacol. 26:521-530. [DOI] [PubMed] [Google Scholar]
- 5.Hellgren, U., I. Johansson, F. Dias, O. Ericsson, J. Stenbeck, and L. Rombo. 1991. Chloroquine resistant Plasmodium falciparum malaria in Guinea-Bissau. Trans. R. Soc. Trop. Med. Hyg. 85:36. [DOI] [PubMed] [Google Scholar]
- 6.Kofoed, P. E., F. Có, P. Johansson, F. Dias, C. Cabral, K. Hedegaard, P. Aaby, and L. Rombo. 2002. Treatment of uncomplicated malaria in children in Guinea-Bissau with chloroquine, quinine, and sulfadoxine-pyrimethamine. Trans. R. Soc. Trop. Med. Hyg. 96:304-309. [DOI] [PubMed] [Google Scholar]
- 7.Kofoed, P. E., F. Lopez, P. Johansson, A. Sandstrom, K. Hedegaard, P. Aaby, and L. Rombo. 2002. Treatment of children with Plasmodium falciparum malaria with chloroquine in Guinea-Bissau. Am. J. Trop. Med. Hyg. 67:28-31. [DOI] [PubMed] [Google Scholar]
- 8.Kofoed, P. E., J. Ursing, A. Poulsen, A. Rodrigues, Y. Bergqvist, P. Aaby, and L. Rombo. 2007. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau: implications for future treatment recommendations. Trans. R. Soc. Trop. Med. Hyg. 101:231-238. [DOI] [PubMed] [Google Scholar]
- 9.Krishna, S., and N. J. White. 1996. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin. Pharmacokinet. 30:263-299. [DOI] [PubMed] [Google Scholar]
- 10.Laufer, M. K., P. C. Thesing, N. D. Eddington, R. Masonga, F. K. Dzinjalamala, S. L. Takala, T. E. Taylor, and C. V. Plowe. 2006. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 355:1959-1966. [DOI] [PubMed] [Google Scholar]
- 11.Lindegårdh, N., M. Forslund, M. D. Green, A. Kaneko, and Y. Bergqvist. 2002. Automated solid-phase extraction for determination of amodiaquine, cloroquine and metabolites in capillary blood on sampling paper by liquid chromatography. Chromatographia 55:5-12. [Google Scholar]
- 12.Looareesuwan, S., N. J. White, P. Chanthavanich, G. Edwards, D. D. Nicholl, C. Bunch, and D. A. Warrell. 1986. Cardiovascular toxicity and distribution kinetics of intravenous chloroquine. Br. J. Clin. Pharmacol. 22:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack, R. B. 1984. 4-Aminoquinolines can be dangerous to your health: chloroquine (Aralen) intoxication. N. C. Med. J. 45:245-246. [PubMed] [Google Scholar]
- 14.Ord, R., N. Alexander, S. Dunyo, R. Hallett, M. Jawara, G. Targett, C. J. Drakeley, and C. J. Sutherland. 2007. Seasonal carriage of pfcrt and pfmdr1 alleles in Gambian Plasmodium falciparum imply reduced fitness of chloroquine-resistant parasites. J. Infect. Dis. 196:1613-1619. [DOI] [PubMed] [Google Scholar]
- 15.Pussard, E., J. P. Lepers, F. Clavier, L. Raharimalala, J. Le Bras, M. Frisk-Holmberg, Y. Bergqvist, and F. Verdier. 1991. Efficacy of a loading dose of oral chloroquine in a 36-hour treatment schedule for uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 35:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues, A., J. A. Schellenberg, P. E. Kofoed, P. Aaby, and B. Greenwood. 2008. Changing pattern of malaria in Bissau, Guinea Bissau. Trop. Med. Int. Health 13:410-417. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez, C. P., W. D. Stein, and M. Lanzer. 2007. Is PfCRT a channel or a carrier? Two competing models explaining chloroquine resistance in Plasmodium falciparum. Trends Parasitol. 23:332-339. [DOI] [PubMed] [Google Scholar]
- 18.Schellenberg, D., C. Menendez, J. J. Aponte, E. Kahigwa, M. Tanner, H. Mshinda, and P. Alonso. 2005. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet 365:1481-1483. [DOI] [PubMed] [Google Scholar]
- 19.Schellenberg, D., C. Menendez, E. Kahigwa, J. Aponte, J. Vidal, M. Tanner, H. Mshinda, and P. Alonso. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357:1471-1477. [DOI] [PubMed] [Google Scholar]
- 20.Smith, E. R., and W. Klein-Schwartz. 2005. Are 1-2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers. J. Emerg. Med. 28:437-443. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland, C. J., C. J. Drakeley, and D. Schellenberg. 2007. How is childhood development of immunity to Plasmodium falciparum enhanced by certain antimalarial interventions? Malar. J. 6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ursing, J., P. E. Kofoed, A. Rodrigues, L. Rombo, and J. P. Gil. 2007. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am. J. Trop. Med. Hyg. 76:844-848. [PubMed] [Google Scholar]
- 23.Ursing, J., B. A. Schmidt, M. Lebbad, P. E. Kofoed, F. Dias, J. P. Gil, and L. Rombo. 2007. Chloroquine resistant P. falciparum prevalence is low and unchanged between 1990 and 2005 in Guinea-Bissau: an effect of high chloroquine dosage? Infect. Genet. Evol. 7:555-561. [DOI] [PubMed] [Google Scholar]
- 24.WHO. 2006. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland.
- 25.Wootton, J. C., X. Feng, M. T. Ferdig, R. A. Cooper, J. Mu, D. I. Baruch, A. J. Magill, and X. Z. Su. 2002. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 418:320-323. [DOI] [PubMed] [Google Scholar]