Abstract
African trypanosomiasis (sleeping sickness), caused by protozoan Trypanosoma brucei species, is a debilitating disease that is lethal if untreated. Available drugs are antiquated, toxic, and compromised by emerging resistance. The indenoisoquinolines are a class of noncamptothecin topoisomerase IB poisons that are under development as anticancer agents. We tested a variety of indenoisoquinolines for their ability to kill T. brucei. Indenoisoquinolines proved trypanocidal at submicromolar concentrations in vitro. Structure-activity analysis yielded motifs that enhanced potency, including alkylamino substitutions on N-6, methoxy groups on C-2 and C-3, and a methylenedioxy bridge between C-8 and C-9. Detailed analysis of eight water-soluble indenoisoquinolines demonstrated that in trypanosomes the compounds inhibited DNA synthesis and acted as topoisomerase poisons. Testing these compounds on L1210 mouse leukemia cells revealed that all eight were more effective against trypanosomes than against mammalian cells. In preliminary in vivo experiments one compound delayed parasitemia and extended survival in mice subjected to a lethal trypanosome challenge. The indenoisoquinolines provide a promising lead for the development of drugs against sleeping sickness.
African trypanosomiasis (sleeping sickness), caused by the protozoan parasite Trypanosoma brucei, is a devastating disease transmitted by tsetse flies and is prevalent throughout sub-Saharan Africa. Lethal if untreated, this infection is responsible for substantial morbidity and mortality (40). The impact of the disease is exacerbated by the paucity of effective drugs, their toxicity, and the emergence of drug resistance (14). This is exemplified by the fact that melarsoprol, an organoarsenical that is the only drug effective against the central nervous system stage of both East and West African sleeping sickness, itself causes 5% mortality and has a disturbing failure rate (14). There is a pressing need to develop new drugs as well as to identify molecular targets for new therapies.
DNA topoisomerases have proven to be effective drug targets in prokaryotic and eukaryotic systems (16, 24, 34). These enzymes catalyze topological changes in DNA and have vital roles in many aspects of nucleic acid metabolism. Based on their mechanism of action, topoisomerases can be classified as type I enzymes, which break a single strand of the DNA helix during the catalytic cycle, and type II enzymes, which make double-stranded breaks. On the basis of primary sequence and reaction mechanism, type I topoisomerases are further subdivided into type IA and type IB (21, 38). Type IB topoisomerases are clinically relevant by virtue of being the target of anticancer camptothecins. The latter are exquisitely specific for topoisomerase IB (38) and belong to a class of agents collectively known as topoisomerase “poisons.” The poisons are a distinct subgroup of topoisomerase inhibitors that bind to and stabilize a normally transient DNA-topoisomerase catalytic intermediate termed the cleavable complex. Persistence of such complexes leads to strand breaks in DNA and cell death, making topoisomerase poisons effective antiproliferative agents (22).
Kinetoplastid topoisomerases have been a focus of much study with a view toward their utilization as drug targets (4, 11, 13). The type IB topoisomerase of T. brucei (and Leishmania donovani, a related kinetoplastid pathogen) is an enzyme singularly distinct from its mammalian counterpart. While all other described type IB topoisomerases are single polypeptide moieties, the enzyme from kinetoplastids is heteromultimeric, composed of two distinct proteins encoded by two independent genes (7). Knock-down studies have revealed that this enzyme is essential for trypanosomes (5), validating its status as a potential drug target. Camptothecin kills trypanosomes by targeting topoisomerase IB (9), and structure-activity studies with camptothecin analogs have illuminated the possibility of generating agents that can selectively target the trypanosomal enzyme (10). Taken together, these aspects make topoisomerase IB poisons a promising source of lead compounds for antitrypanosomal drug development.
Indenoisoquinolines, a noncamptothecin class of synthetic compounds (Fig. 1), were originally identified in a search for anticancer agents. In the 60-cell-line National Cancer Institute anticancer drug screen, indenoisoquinolines demonstrated a cell-line-specific cytotoxicity profile consistent with topoisomerase IB poisons, and they can trap topoisomerase IB-DNA covalent complexes (19, 20, 36). Some of the indenoisoquinolines have the ability to bind and intercalate into DNA in the absence of the enzyme (30, 35, 36), and others may even interact directly with the mammalian topoisomerase IB protein at high drug concentrations (29). We have evaluated a battery of indenoisoquinolines (26-28, 30) against T. brucei and we find that they have potent antitrypanosomal activities in vitro, inhibit nucleic acid synthesis in the parasite, act as topoisomerase poisons within the cell, and show preliminary evidence of efficacy in mice challenged with trypanosomes.
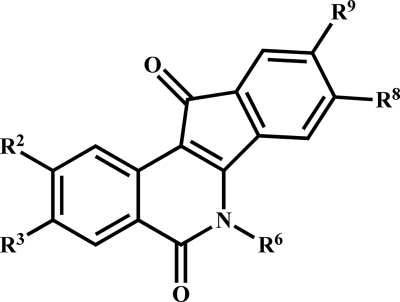
FIG. 1.
The indenoisoquinoline scaffold. Structure-activity analysis was performed with substitutions at the numbered positions.
MATERIALS AND METHODS
Cells, culture conditions, and test compounds.
Bloodstream-form Trypanosoma brucei brucei 427 cells (doubling time, 6 to 8 h) were maintained in HMI-9 medium containing 10% fetal bovine serum and 10% Serum Plus. Mouse leukemia L1210 cells (ATCC; doubling time, 20 to 24 h) were maintained in RPMI 1640 containing 15% fetal bovine serum. Parasites and mammalian cells were cultured at 37°C with 5% CO2. Logarithmically growing cells (densities of 1 × 105 to 5 × 105 cells/ml) were used for all assays. Test compounds were stored desiccated at −20°C and were dissolved in dimethyl sulfoxide (DMSO) or water just prior to use. Diminazene aceturate (Berenil) was purchased from Sigma (St. Louis, MO).
Cytotoxicity assay for trypanosomes and L1210 cells.
Cytotoxicity was measured using an acid phosphatase-based 96-well plate assay (8). Briefly, T. brucei cells (100 μl; 2 × 105 cells/ml) were incubated with 100 μl medium containing solvent or drug for 24 h. Final DMSO concentrations did not exceed 0.1%. Acid phosphatase activity was measured by adding 20 μl lysis buffer (20 mg/ml p-nitrophenyl phosphate in 1 M sodium acetate, pH 5.5, 1% Triton X-100) and incubating for 5 h at 37°C. Reactions were terminated with 10 μl 1 N NaOH, and absorbance was measured at 405 nm. Lethality was confirmed by microscopic examination of drug-treated cells prior to addition of lysis/substrate solution. L1210 cells (100 μl; 1.4 × 105 cells/ml) were incubated with 100 μl medium containing drug or solvent for 48 h in 96-well plates (8) and processed as described above. Prior to lysis, aliquots of drug-treated cells were subjected to the trypan blue exclusion test to confirm lethality (12). Each concentration was assayed in quadruplicate. Curve fitting and 50% effective concentration (EC50) determinations were performed using the Emax model (17).
Thymidine incorporation assay.
Trypanosomes (500 μl; 2 × 106 cells/ml) were incubated for 3 h with [3H]thymidine (100 μCi/ml; 20 Ci/mmol) in the presence of drug or solvent in thymidine-free HMI-9 (9). Cells were deposited onto filter paper (blotting paper 703; VWR Scientific Products), and nucleic acids were precipitated using ice-cold 5% trichloroacetic acid. Filters were washed with ethanol, and radioactivity was quantified by liquid scintillation. Camptothecin (10 μM) was used as a positive control. Inhibition of DNA synthesis was measured via the percent inhibition of [3H]thymidine incorporation (disintegrations per minute [dpm]), expressed as follows: [1 − (dpmdrug/dpmcontrol)] × 100. The solvents (water for the indenoisoquinolines and DMSO for camptothecin) had no effect on [3H]thymidine incorporation. Experiments were run in triplicate.
Assay for cleavable complexes.
Cleavable complex formation in situ was measured as described previously (9, 37), with the following modification. After addition of KCl, the reaction mixture was incubated on ice for 60 min. The precipitate was harvested by filtering on glass fiber paper (GF/C; Brandel Inc., MD) prewetted with wash buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA, 100 mM KCl), washed (three times with 4 ml of wash buffer), and air dried. Total incorporation of radioactivity into DNA was measured by spotting 50 μl of labeled cell suspension onto filter paper, precipitating nucleic acids using 5% trichloroacetic acid, and counting by liquid scintillation. All assays included 10 μM camptothecin and solvent controls. Cleavable complex formation, as a percentage of total labeled DNA, was calculated as follows: [(dpm in K-SDSdrug − dpm in K-SDSsolvent)/(dpmtotal incorporation)] × 100 (K-SDS is the K-sodium dodecyl sulfate assay). Each experiment was run in triplicate.
Efficacy in vivo.
This work was conducted with approval of the Johns Hopkins University Institutional Animal Care and Use Committee. Groups of two 6-week-old female CD1 mice were untreated or injected intraperitoneally with (i) a single dose of 50 mg/kg test indenoisoquinoline (4 mg/ml for compounds 20 and 23 and 2 mg/ml for compound 12, all in 0.9% saline) (27) or (ii) 3.5 mg/kg Berenil (0.7 mg/ml in 0.9% saline) (41). Three h after dosing, mice were challenged with 5 × 105 Trypanosoma brucei (5 × 106 cells/ml, intraperitoneal) (2). Parasitemia was monitored every 24 h by sampling tail vein blood and quantifying parasites in a hemocytometer. Mice in evident distress were euthanized and considered to have succumbed to the challenge.
RESULTS AND DISCUSSION
Activity of indenoisoquinolines against trypanosomes.
We tested a spectrum of compounds with various substitutions on a core indenoisoquinoline nucleus (Fig. 1) for their ability to kill T. brucei. Results from the cytotoxicity assay revealed that the test compounds were trypanocidal, with micromolar or submicromolar EC50s (see Tables 1, 2, and 3). Such potency compares favorably with that of camptothecin (EC50, 1.5 μM) and to the clinically utilized antitrypanosomal drugs pentamidine (EC50, 0.014 μM) and Berenil (EC50, 0.094 μM) (8).
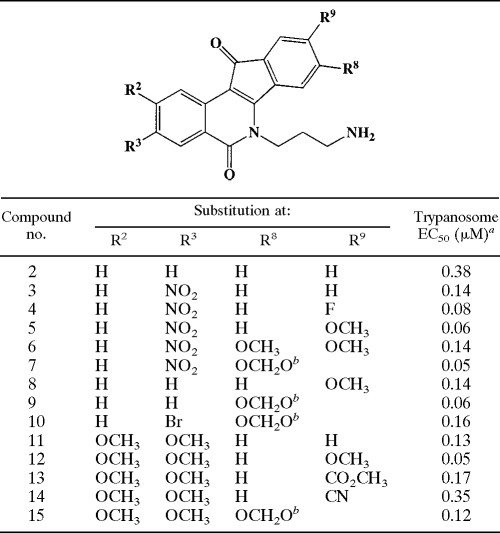
TABLE 1.
Structure-activity relationship for N-6 3-aminopropyl indenoisoquinolines
aFor a given compound, EC50s differed by less than 27%. R2 values for dose-response curves all exceeded 0.98, and the coefficient of variation for quadruplicate determinations at a given concentration was less than 12%.
bThe OCH2O moiety bridged positions R8 and R9.
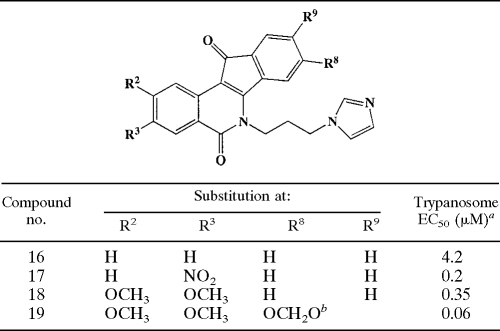
TABLE 2.
Structure-activity relationship for N-6 3-imidazolylprolyl indenoisoquinolines
aDifferences between the average EC50s were less than 18%. R2 values obtained for dose-response curves all exceeded 0.97, and the coefficient of variation for quadruplicate determinations at any concentration was less than 9%.
bThe OCH2O moiety bridged positions R8 and R9.
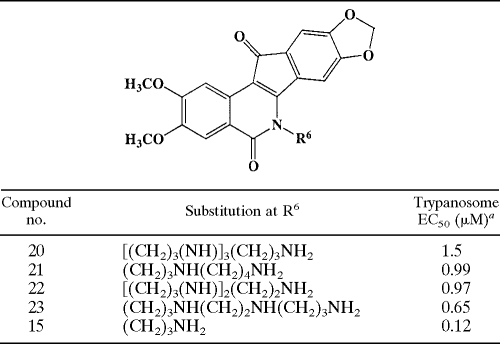
TABLE 3.
Effect of various alkylamine repeats N-6 on trypanosome killing
aDifferences between the average EC50s were less than 16%. R2 values obtained for dose-response curves all exceeded 0.98. The coefficient of variation for quadruplicate determinations at a given concentration was less than 12%.
Structure-activity relationships in trypanocidal indenoisoquinolines.
We examined the EC50s for trypanocidal indenoisoquinolines in the context of molecular structure. Initial characterization revealed that while the core moiety (Fig. 1) was essentially inactive (<20% kill at 10 μM), the single modification of an alkylamine substituent at N-6 conferred robust activity (Table 1, compound 2). Substitution of electron-withdrawing groups on other ring positions of the N-6-substituted core further improved activity. Potency was markedly enhanced by an 8,9-methylenedioxy bridge (compare compound 3 versus compound 7 and compound 2 versus compound 9). The importance of the methylenedioxy bridge is reminiscent of its role in boosting the potency of camptothecin analogs (10) and is consistent with the notion that indenoisoquinolines act via topoisomerase IB. The presence of methoxy groups at C-2, C-3, and C-9 also contribute to trypanosome-killing activity (compare compound 2 versus 11, compound 3 versus 5, and compound 8 versus 12).
Replacement of the terminal NH2 on the N-6 substituent with an imidazole ring reduced potency (compound 16 versus 2). However, the general structure-activity relationships deduced from the N-6-aminopropyl series also held true in the N-6-(3-imidazolyl-1-propyl) series (Table 2). The nature of the N-6 substituent influenced trypanocidal activity, such that the 3-aminopropyl-substituted N-6 compound (number 15) was more active than analogs with other varied alkylamine repeats (Table 3).
All 2,3-dimethoxy-substituted compounds were water soluble to an appreciable extent; in fact, the motif was predictive of aqueous solubility. Water solubility is a desirable quality for a molecule in the context of drug development. We therefore utilized this characteristic to select a subset of analogs for further analysis. Eight water-soluble compounds (limit of solubility varying from 2.5 mM to 10 mM) (Table 4), with antitrypanosomal EC50s ranging widely from 1.5 μM to 50 nM, were selected for detailed characterization.
TABLE 4.
Activities of water-soluble indenoisoquinolines against trypanosomes and L1210 cells
| Compound no. | EC50 (μM)
|
In trypanosomes
|
|||
|---|---|---|---|---|---|
| Trypanosomesa | L1210b | Ratioc | % Inhibition of DNA synthesisd | % Cleavable complexese | |
| 20 | 1.5 | 2.8 | 1.9 | 34 ± 4 | 10 ± 3 |
| 21 | 0.99 | 4.3 | 4.3 | 56 ± 2 | 7 ± 1 |
| 23 | 0.65 | 2.6 | 4.0 | 44 ± 3 | 11 ± 1 |
| 14 | 0.35 | 0.97 | 2.8 | 69 ± 5 | 3 ± 1 |
| 13 | 0.17 | 0.42 | 2.5 | 66 ± 5 | 12 ± 2 |
| 11 | 0.13 | 0.51 | 4.3 | 67 ± 11 | 4 ± 2 |
| 15 | 0.12 | 0.25 | 2.1 | 61 ± 3 | 4 ± 1 |
| 12 | 0.05 | 0.28 | 5.6 | 68 ± 7 | 3 ± 1 |
R2 values for all dose-response curves were greater than 0.99, and the coefficient of variation for quadruplicate determinations at a given concentration was less than 20%.
Ratio of EC50 of L1210 and EC50 of trypanosomes. The 95% confidence intervals around the antitrypanosomal and anti-L1210 EC50s did not overlap.
Percent inhibition of incorporation of [3H]thymidine into trichloroacetic acid-precipitable counts by a 5 μM concentration of the compound; means ± standard deviations from three independent experiments are shown. Camptothecin at 10 μM (n = 3) yielded 27 ± 5% inhibition.
Percentage of total incorporated [3H]thymidine trapped in K-SDS precipitate after treatment with a 5 μM concentration of the compound; data are means ± standard deviations from three independent experiments. Untreated or DMSO-treated cells yielded 17 ± 2% cleavable complexes, a value subtracted from results for drug-treated cells. Camptothecin at 10 μM (n = 5) trapped 61 ± 8% of radiolabeled DNA.
Activity against L1210 cells.
To provide a uniform basis for comparison in these studies, we assayed the water-soluble compounds against mouse leukemia L1210 cells. These cells are highly susceptible to camptothecin, have been used extensively for studying topoisomerase IB-targeting agents, and are a benchmark for comparative screening of antitrypanosomal candidates (10, 23, 39). Although all eight compounds were active against L1210 cells, they were systematically, if modestly, more potent against trypanosomes (Table 4). The structure-activity relationships governing antitrypanosomal potency appear to be paralleled in L1210 cells. It has been demonstrated that nonmalignant cells are generally less susceptible to topoisomerase-targeting agents than are cancer cells (32, 33), suggesting that the selectivity of these indenoisoquinolines for trypanosomes will be greater compared to normal cells. Furthermore, the indenoisoquinoline derivatives tested here were originally synthesized with the aim of maximizing mammalian cell killing. The presence of consistent, albeit low, selectivity toward trypanosomes in this biased sample suggests that screening a naïve indenoisoquinoline library may yield agents with much greater efficacy against parasites compared to mammalian cells.
Inhibition of trypanosome DNA synthesis by indenoisoquinolines.
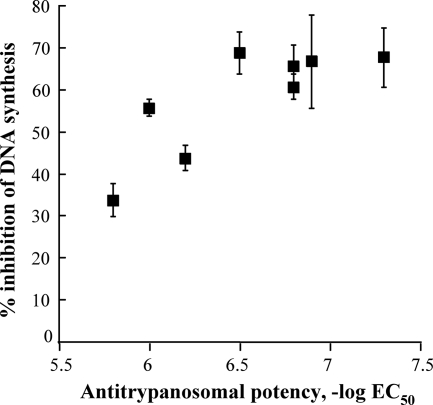
Inhibition of nucleic acid synthesis is a consequence of targeting topoisomerase IB in trypanosomes (9). We tested the ability of the water-soluble indenoisoquinolines to inhibit DNA synthesis in trypanosomes and found that the series effectively inhibited [3H]thymidine incorporation (Table 4). Additionally, cytotoxicity correlated with inhibition of DNA synthesis (Fig. 2). This is consistent with the notion that inhibition of DNA synthesis, perhaps by targeting of topoisomerase IB, is a mediator of cell killing. Notably, the indenoisoquinolines were more potent than camptothecin in both cytotoxicity and inhibition of DNA synthesis. The apparent maximum inhibition of ∼70% for these compounds may reflect ongoing topoisomerase IB-independent DNA processes, such as repair or mitochondrial DNA synthesis.
FIG. 2.
Trypanocidal activity is related to inhibition of DNA synthesis. Each point is a single compound whose ability to inhibit DNA synthesis was evaluated at 5 μM. Antitrypanosomal activity is expressed on a molar basis. For the concurrent positive control, camptothecin, the −log EC50 was 5.8 and inhibition of DNA synthesis was 27 ± 5% at 10 μM. Control cells incorporated 24,998 ± 1,729 dpm (n = 6) under assay conditions. Error bars, 1 standard deviation.
Formation of cleavable complexes by indenoisoquinolines.
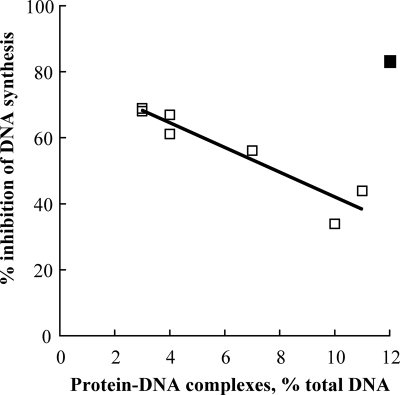
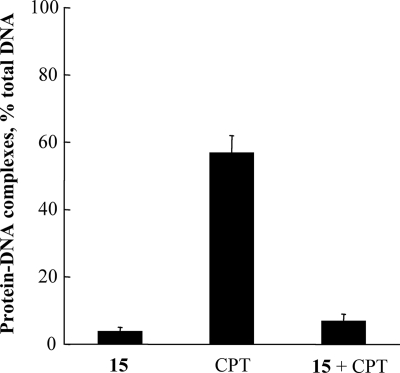
We utilized the K-SDS method to assess the ability of indenoisoquinolines to poison topoisomerase in situ (18). In this method, intracellular topoisomerase-DNA cleavable complexes are trapped by the addition of SDS, then detergent and accompanying denatured proteins are precipitated with potassium, and radiolabeled DNA in the precipitate indicates the presence of covalent protein-DNA adducts. In trypanosomes the indenoisoquinolines are indeed topoisomerase poisons (Table 4). Surprisingly, however, their potency in this assay did not exceed ∼12% (Table 4; camptothecin control, 61%). Based on their general superiority to camptothecin in both the cytotoxicity and DNA inhibition assays, this value was unexpectedly low. Furthermore, although dose-response analysis for compound 20 revealed an increase in cleavable complex formation as drug concentration increased up to 5 μM (data not shown), the ability of these compounds to trap complexes did not correlate with their ability to inhibit DNA synthesis (Fig. 3). Curiously, in fact, for seven of eight compounds tested, the ability to form cleavable complexes correlated inversely with inhibition of nucleic acid synthesis (Fig. 3; compound 13, with an ester substituent at C-9, does not follow this trend). The disproportionately low poisoning activity and lack of coincidence between poisoning and cytotoxicity suggest that the stabilization of topoisomerase IB-DNA complexes is not the sole determinant of killing. Perhaps the direct interaction of indenoisoquinolines with DNA (30, 35) prevents the topoisomerase from binding to its substrate, thus abrogating their effectiveness as poisons. This phenomenon was previously demonstrated with minor groove binders and mammalian topoisomerase IB (25). We tested this hypothesis by assaying the ability of camptothecin to promote cleavable complex formation in situ after the cells were preincubated with indenoisoquinoline compound 15 and found that indenoisoquinoline pretreatment blocked the poisoning activity of camptothecin (Fig. 4).
FIG. 3.
Inhibition of DNA synthesis is inversely related to stabilization of cleavable complexes in the cell. Each point is a single compound whose ability to promote cleavable complex formation in situ was evaluated at 5 μM. If compound 13 (▪) is excluded from the analysis, the fitted line follows the equation y = 80 − 3.8x, and R2 = 0.88. In these experiments, the positive control camptothecin at 10 μM trapped 61 ± 8% of radiolabeled DNA in covalent protein-DNA adducts and inhibited DNA synthesis at 27 ± 5%. Standard deviation values are reported in Table 4.
FIG. 4.
Indenoisoquinoline compound 15 prevents camptothecin (CPT)-induced cleavable complex formation. Trypanosomes were metabolically labeled with [3H]thymidine, washed, and aliquoted for treatment with 5 μM compound 15 or 10 μM camptothecin for 30 min, or with 5 μM compound 15 for 5 min prior to addition of 10 μM CPT, followed by an additional 25-min incubation. Results are means of three independent experiments ± standard deviations.
The lower-than-expected extent of poisoning of trypanosome topoisomerase could be attributed to multiple factors. Relative to mammalian cells, African trypanosomes have a unique heteromultimeric topoisomerase IB (7) and a substantially more AT-rich genome (6) that is packaged differently (15). Thus, decreased affinity for the topoisomerase-DNA binary complex, and/or increased accessibility of the DNA, could explain this finding. Direct interaction with DNA implies that the compounds may also interfere with other DNA metabolic processes, explaining their inhibition of replication. Indeed, although indenoisoquinolines have been demonstrated to be topoisomerase IB poisons in mammalian cells (1, 20), the greater-than-expected killing activity against T. brucei makes it formally possible that cleavable complexes detected in the K-SDS assay may contain DNA adducts with other topoisomerases. Additionally, while the N-6-polyamine substituent may well enhance transport into the trypanosome, the demonstrated activity of other polyamine analogs against T. brucei (31) permits speculation that these N-6-substituted indenoisoquinolines may also interfere with the polyamine metabolic pathway, a proven drug target in these parasites (3).
Preliminary survey of efficacy in mice.
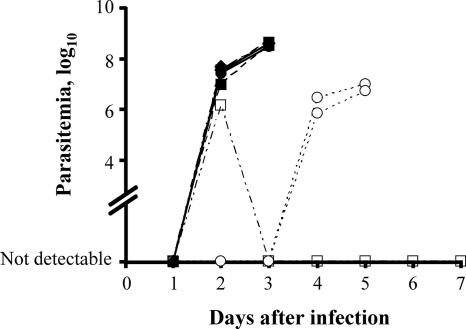
To assess further the potential of indenoisoquinolines as antitrypanosomal agents, three of the water-soluble compounds with a range of activity in vitro (compound 20, EC50 of 1.5 μM; compound 23, EC50 of 0.65 μM; compound 12, EC50 of 0.05 μM) were tested for their ability to protect mice against a lethal T. brucei challenge. As expected, by day 2 after infection the untreated mice developed a rapidly progressing patent infection (2) and the positive controls treated with Berenil developed a transient parasitemia that remained below the limit of detection after day 3 (Fig. 5). Indenoisoquinolines compound 20 and 23 had no apparent effect on progression of the infection. However, treatment with compound 12, the most potent compound in vitro, conferred some protection against trypanosome infection. In these animals, parasites were undetectable in the blood until day 4 postinfection (Fig. 5). Once parasites appeared the infection progressed normally. The drugs themselves caused no apparent ill effects in the animals. This preliminary experiment has a number of obvious limitations, including a sample size that precludes statistical comparison and the almost-certainly suboptimal single-dose regimen. Nevertheless, the results of this proof-of-concept study are promising.
FIG. 5.
In vivo efficacies of indenoisoquinolines. Mice were dosed with indenosioquinoline compound 12 (open circles), compound 20 (filled circles), or compound 23 (filled squares) at 50 mg/kg and challenged with 5 × 105 T. brucei. Berenil (open squares) served as a positive drug control. Untreated animals (filled diamonds) and mice dosed with compounds 20 or 23 had detectable parasitemia 2 days after infection and succumbed by day 4. Indenoisoquinoline compound 12 delayed the appearance of parasites until 4 days postinfection and increased survival to 6 days. The lower limit of detection of parasitemia was log10 3 (103 cells/ml).
In summary, we found that indenoisoquinolines have potent antitrypanosomal activity in vitro and demonstrable activity in mice. Their killing action may involve multiple molecular mechanisms. They clearly stabilize topoisomerase-DNA complexes in situ and may also impede topoisomerase binding to DNA. These agents markedly inhibit DNA synthesis by interfering with topoisomerase and possibly other DNA-metabolizing enzymes. Multiple mechanisms of action against trypanosomes would be an attractive feature, since the potential for drug resistance is reduced. Indenoisoquinolines are a promising lead for development of a new, much-needed, antitrypanosomal therapy.
Acknowledgments
The assistance of Chad Slawson and other members of the Hart laboratory is gratefully acknowledged. We thank Suji Xie for technical support, Paul Englund and So Hee Lee for assistance with animal experiments, and Jane Scocca and David Meyers for their thoughtful comments on the manuscript.
This study was funded by NIH grants AI028855 and UO1 CA89566.
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Antony, S., K. K. Agama, Z. H. Miao, K. Takagi, M. H. Wright, A. I. Robles, L. Varticovski, M. Nagarajan, A. Morrell, M. Cushman, and Y. Pommier. 2007. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topoisomerase I cleavage complexes and overcome multidrug resistance. Cancer Res. 67:10397-10405. [DOI] [PubMed] [Google Scholar]
- 2.Bacchi, C. J., M. Vargas, D. Rattendi, B. Goldberg, and W. Zhou. 1998. Antitrypanosomal activity of a new triazine derivative, SIPI 1029, in vitro and in model infections. Antimicrob. Agents Chemother. 42:2718-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacchi, C. J., and N. Yarlett. 2002. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2:553-563. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi, R. P., and T. A. Shapiro. 2003. DNA topoisomerases as targets for antiprotozoal therapy. Mini Rev. Med. Chem. 3:597-608. [DOI] [PubMed] [Google Scholar]
- 5.Bakshi, R. P., and T. A. Shapiro. 2004. RNA interference of Trypanosoma brucei topoisomerase IB: both subunits are essential. Mol. Biochem. Parasitol. 136:249-255. [DOI] [PubMed] [Google Scholar]
- 6.Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, et al. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309:416-422. [DOI] [PubMed] [Google Scholar]
- 7.Bodley, A. L., A. K. Chakraborty, S. Xie, C. Burri, and T. A. Shapiro. 2003. An unusual type IB topoisomerase from African trypanosomes. Proc. Natl. Acad. Sci. USA 100:7539-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodley, A. L., M. W. McGarry, and T. A. Shapiro. 1995. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J. Infect. Dis. 172:1157-1159. [DOI] [PubMed] [Google Scholar]
- 9.Bodley, A. L., and T. A. Shapiro. 1995. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc. Natl. Acad. Sci. USA 92:3726-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodley, A. L., M. C. Wani, M. E. Wall, and T. A. Shapiro. 1995. Antitrypanosomal activity of camptothecin analogs. Structure-activity correlations. Biochem. Pharmacol. 50:937-942. [DOI] [PubMed] [Google Scholar]
- 11.Burri, C., A. L. Bodley, and T. A. Shapiro. 1996. Topoisomerases in kinetoplastids. Parasitol. Today 12:226-231. [DOI] [PubMed] [Google Scholar]
- 12.Cook, J. A., and J. B. Mitchell. 1989. Viability measurements in mammalian cell systems. Anal. Biochem. 179:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Das, A., A. Dasgupta, T. Sengupta, and H. K. Majumder. 2004. Topoisomerases of kinetoplastid parasites as potential chemotherapeutic targets. Trends Parasitol. 20:381-387. [DOI] [PubMed] [Google Scholar]
- 14.Docampo, R., and S. N. Moreno. 2003. Current chemotherapy of human African trypanosomiasis. Parasitol. Res. 90(Supp. 1):S10-S13. [DOI] [PubMed] [Google Scholar]
- 15.Ersfeld, K., S. E. Melville, and K. Gull. 1999. Nuclear and genome organization of Trypanosoma brucei. Parasitol. Today 15:58-63. [DOI] [PubMed] [Google Scholar]
- 16.Fortune, J. M., and N. Osheroff. 2000. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog. Nucleic Acid Res. Mol. Biol. 64:221-253. [DOI] [PubMed] [Google Scholar]
- 17.Holford, N. H., and L. B. Sheiner. 1981. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin. Pharmacokinet. 6:429-453. [DOI] [PubMed] [Google Scholar]
- 18.Hsiang, Y. H., R. Hertzberg, S. Hecht, and L. F. Liu. 1985. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 260:14873-14878. [PubMed] [Google Scholar]
- 19.Ioanoviciu, A., S. Antony, Y. Pommier, B. L. Staker, L. Stewart, and M. Cushman. 2005. Synthesis and mechanism of action studies of a series of norindenoisoquinoline topoisomerase I poisons reveal an inhibitor with a flipped orientation in the ternary DNA-enzyme-inhibitor complex as determined by X-ray crystallographic analysis. J. Med. Chem. 48:4803-4814. [DOI] [PubMed] [Google Scholar]
- 20.Kohlhagen, G., K. D. Paull, M. Cushman, P. Nagafuji, and Y. Pommier. 1998. Protein-linked DNA strand breaks induced by NSC 314622, a novel noncamptothecin topoisomerase I poison. Mol. Pharmacol. 54:50-58. [DOI] [PubMed] [Google Scholar]
- 21.Leppard, J. B., and J. J. Champoux. 2005. Human DNA topoisomerase I: relaxation, roles, and damage control. Chromosoma 114:75-85. [DOI] [PubMed] [Google Scholar]
- 22.Liu, L. F., S. D. Desai, T. K. Li, Y. Mao, M. Sun, and S. P. Sim. 2000. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 922:1-10. [DOI] [PubMed] [Google Scholar]
- 23.Mattern, M. R., S. M. Mong, H. F. Bartus, C. K. Mirabelli, S. T. Crooke, and R. K. Johnson. 1987. Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured L1210 cells. Cancer Res. 47:1793-1798. [PubMed] [Google Scholar]
- 24.Maxwell, A. 1999. DNA gyrase as a drug target. Biochem. Soc. Trans. 27:48-53. [DOI] [PubMed] [Google Scholar]
- 25.McHugh, M. M., R. D. Sigmund, and T. A. Beerman. 1990. Effects of minor groove binding drugs on camptothecin-induced DNA lesions in L1210 nuclei. Biochem. Pharmacol. 39:707-714. [DOI] [PubMed] [Google Scholar]
- 26.Morrell, A., S. Antony, G. Kohlhagen, Y. Pommier, and M. Cushman. 2004. Synthesis of nitrated indenoisoquinolines as topoisomerase I inhibitors. Bioorg. Med. Chem. Lett. 14:3659-3663. [DOI] [PubMed] [Google Scholar]
- 27.Morrell, A., M. Jayaraman, M. Nagarajan, B. M. Fox, M. R. Meckley, A. Ioanoviciu, Y. Pommier, S. Antony, M. Hollingshead, and M. Cushman. 2006. Evaluation of indenoisoquinoline topoisomerase I inhibitors using a hollow fiber assay. Bioorg. Med. Chem. Lett. 16:4395-4399. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan, M., A. Morrell, B. C. Fort, M. R. Meckley, S. Antony, G. Kohlhagen, Y. Pommier, and M. Cushman. 2004. Synthesis and anticancer activity of simplified indenoisoquinoline topoisomerase I inhibitors lacking substituents on the aromatic rings. J. Med. Chem. 47:5651-5661. [DOI] [PubMed] [Google Scholar]
- 29.Nagarajan, M., A. Morrell, A. Ioanoviciu, S. Antony, G. Kohlhagen, K. Agama, M. Hollingshead, Y. Pommier, and M. Cushman. 2006. Synthesis and evaluation of indenoisoquinoline topoisomerase I inhibitors substituted with nitrogen heterocycles. J. Med. Chem. 49:6283-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagarajan, M., X. Xiao, S. Antony, G. Kohlhagen, Y. Pommier, and M. Cushman. 2003. Design, synthesis, and biological evaluation of indenoisoquinoline topoisomerase I inhibitors featuring polyamine side chains on the lactam nitrogen. J. Med. Chem. 46:5712-5724. [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan, M. C., Q. Zhou, Z. Li, T. B. Durham, D. Rattendi, S. Lane, and C. J. Bacchi. 1997. Polyamine derivatives as inhibitors of trypanothione reductase and assessment of their trypanocidal activities. Bioorg. Med. Chem. 5:2145-2155. [DOI] [PubMed] [Google Scholar]
- 32.Pantazis, P., J. A. Early, A. J. Kozielski, J. T. Mendoza, H. R. Hinz, and B. C. Giovanella. 1993. Regression of human breast carcinoma tumors in immunodeficient mice treated with 9-nitrocamptothecin: differential response of nontumorigenic and tumorigenic human breast cells in vitro. Cancer Res. 53:1577-1582. [PubMed] [Google Scholar]
- 33.Pantazis, P., H. R. Hinz, J. T. Mendoza, A. J. Kozielski, L. J. Williams, Jr., J. S. Stehlin, Jr., and B. C. Giovanella. 1992. Complete inhibition of growth followed by death of human malignant melanoma cells in vitro and regression of human melanoma xenografts in immunodeficient mice induced by camptothecins. Cancer Res. 52:3980-3987. [PubMed] [Google Scholar]
- 34.Pommier, Y., P. Pourquier, Y. Fan, and D. Strumberg. 1998. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim. Biophys. Acta 1400:83-105. [DOI] [PubMed] [Google Scholar]
- 35.Staker, B. L., M. D. Feese, M. Cushman, Y. Pommier, D. Zembower, L. Stewart, and A. B. Burgin. 2005. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 48:2336-2345. [DOI] [PubMed] [Google Scholar]
- 36.Strumberg, D., Y. Pommier, K. Paull, M. Jayaraman, P. Nagafuji, and M. Cushman. 1999. Synthesis of cytotoxic indenoisoquinoline topoisomerase I poisons. J. Med. Chem. 42:446-457. [DOI] [PubMed] [Google Scholar]
- 37.Trask, D. K., J. A. DiDonato, and M. T. Muller. 1984. Rapid detection and isolation of covalent DNA/protein complexes: application to topoisomerase I and II. EMBO J. 3:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, J. C. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3:430-440. [DOI] [PubMed] [Google Scholar]
- 39.Wani, M. C., A. W. Nicholas, G. Manikumar, and M. E. Wall. 1987. Plant antitumor agents. 25. Total synthesis and antileukemic activity of ring A substituted camptothecin analogues. Structure-activity correlations. J. Med. Chem. 30:1774-1779. [DOI] [PubMed] [Google Scholar]
- 40.Welburn, S. C., and M. Odiit. 2002. Recent developments in human African trypanosomiasis. Curr. Opin. Infect. Dis. 15:477-484. [DOI] [PubMed] [Google Scholar]
- 41.Witola, W. H., N. Inoue, K. Ohashi, and M. Onuma. 2004. RNA-interference silencing of the adenosine transporter-1 gene in Trypanosoma evansi confers resistance to diminazene aceturate. Exp. Parasitol. 107:47-57. [DOI] [PubMed] [Google Scholar]