The successful management of invasive fungal infections continues to pose a difficult challenge to clinicians. As the population of at-risk immunocompromised patients increases, the incidence of invasive mycosis has risen in parallel (57). Despite recent advances in antifungal pharmacology, the morbidity and mortality due to invasive fungal infections remain unacceptably high.
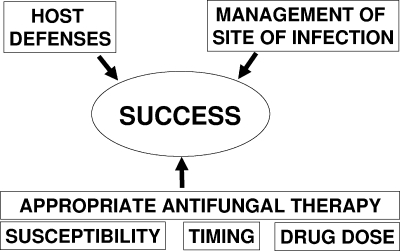
Numerous factors determine the outcome of these infections, including the host immune state, pathogen variables including drug susceptibility, location of the infection, timing of diagnosis relative to initiation of antifungal therapy, management of the infection source (e.g., surgery or vascular catheter removal), and effective administration of the appropriate dose of the most potent and safe antifungal drug (Fig. 1). Few of these variables are under the control of the clinician. The choices of antifungal drug and dosing regimen are among the factors which the clinician can dictate. However, even if the appropriate drug and regimen are initiated, drug exposure at the site of infection may be inadequate due to pharmacokinetic variability and may contribute to treatment failure. Conversely, antifungal exposures may exceed those anticipated and may result in toxicity. Many antifungal drugs exhibit marked variability in drug blood concentrations due to inconsistent absorption, metabolism, elimination, or interaction with concomitant medications. An understanding of the pharmacokinetics and pharmacodynamics of these drugs has been demonstrated to be important to optimize drug choice and dosing regimen design. One tool to detect drug exposures outside of the therapeutic window is monitoring of drug concentration in blood. The utility of this tool to inform drug choice and dosing decisions is being increasingly explored.
FIG. 1.
Determinants for outcome of invasive mycoses.
Several factors must be considered in determining the role of therapeutic drug monitoring in patient management (32, 86, 88). An accurate, rapid, and cost-effective drug assay must be readily available to the clinician. Two pharmacological features then determine the relevance of monitoring of concentrations of drug in blood. The foremost variable is an unpredictable drug dose-exposure relationship. Next, there must be a clear relationship between concentrations of drug in blood and either toxicity or treatment efficacy. Consideration of these pharmacological variables for available antifungal compounds is the focus of this review.
ANTIFUNGAL DRUG MONITORING ASSAY: VALIDATION, COST, AND AVAILABILITY
Validated assays have been developed for each of the commonly used antifungal drugs (Table 1) (61, 69). These assays most often include either a microbiological or chromatographic (high-performance liquid chromatography [HPLC] or liquid chromatography-mass spectrometry [LC-MS]) assay. The most commonly utilized method is HPLC or LC-MS due to the enhanced sensitivity and the reduced time necessary to complete the assay. In most cases, both microbiological and chromatographic methods provide similar results. An exception to this rule is for the triazole antifungal itraconazole. Microbiological assay results for itraconazole are two to three times higher than those observed with HPLC (29, 32). This discrepancy is due to the enhanced microbiological activity of the itraconazole metabolite (hydroxyitraconazole) relative to that of the parent molecule. For all other available antifungals, there are either no microbiologically active metabolites or none of clinical significance. One clinical scenario that can impact the utility of microbiological assays is the use of two or more antifungals in combination. Unless the assay organism is susceptible to only one of the antifungals being used, the assay measurement will reflect the activity from both drugs. While pharmacodynamic investigations have demonstrated the importance of considering free or non-protein-bound concentrations, the assays described above measure both bound and unbound drug. All reference to drug concentrations in this review refers to the total drug concentration unless otherwise specified.
TABLE 1.
Analytical methods for monitoring of antifungal blood levels
| Method | Pros of method | Cons of method | Detection of drug by reported methods
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flucyto-sine | Flucon-azole | Itracon-azole | Posacon-azole | Voricon-azole | Ampho-tericin-B | Anidula-fungin | Caspo-fungin | Mica-fungin | |||
| Chromatographic (HPLC and LC-MS) | Reference methods, high sensitivity, high specificity, rapid analysis | Expensive equipment and specialized technicians, time to results of up to 1 wk for central laboratories | + | + | + | + | + | + | + | + | + |
| Microbiological (bioassay) | Inexpensive, clinically relevant concn range, assessment of microbiologically active metabolites, applicable in local microbiology laboratory with time to result of 24 h | Interferences in combination antifungal therapy, cross-validation with chromatographic method needed | + | + | + | + | + | + | − | + | − |
Unfortunately, the pharmacoeconomic impact of antifungal therapeutic drug monitoring has not been formally investigated. However, the cost of these assays is less than the cost of a single day of antifungal therapy with one of the new compounds and certainly less than that of any patient complication associated with inappropriate antifungal drug exposures.
At present, there are a limited number of specialized clinical laboratories in the United States and Europe that perform these assays. Thus, the time between sample collection and a laboratory result may approach 1 week in some circumstances. However, several studies have demonstrated clinical utility with drug and dosing regimen changes in this window of time (38, 63, 82, 83). One might speculate that favorable outcomes will be more common by reducing the time necessary for measurement reporting. The development of additional and local laboratories may be necessary for the clinical utility of antifungal blood concentration monitoring to achieve its full potential in routine patient care.
SPECIFIC ANTIFUNGAL DRUGS
5-FC.
Flucytosine (5-FC) is a pyrimidine analog that was one of the first antifungal compounds developed. The drug has broad-spectrum activity against Candida species and Cryptococcus neoformans. Clinical use of 5-FC is limited mainly to combination therapy with another antifungal, amphotericin B, for the treatment of cryptococcal meningitis (9, 28). Use in other situations has been limited due to clinician concerns regarding drug toxicity and resistance development with monotherapy administration (35, 67, 73).
The compound is available as an intravenous formulation (only in Europe) and as an oral formulation that is reliably and nearly completely absorbed (67, 98). The timing of monitoring of this antifungal, which has a relatively rapid elimination half-life (3 to 6 h), has been traditionally within 1 to 3 h of administration. The drug dose is weight based (150 mg/kg/day) and administered in four divided doses. However, wide inter-and intrapatient pharmacokinetic variabilities were recognized during initial drug development and recently confirmed in a report of a large clinical experience (67). The predominant variable accounting for these differences is variation in the renal elimination of 5-FC (21, 22). Much of this variation is due to nephrotoxicity associated with the concomitant administration of amphotericin B. A recent report of more than 1,000 5-FC serum concentrations for 233 patients with invasive fungal infections demonstrated marked pharmacokinetic variability (67). In this large cohort, monitoring identified “therapeutic” peak concentrations in only 20% of patients. 5-FC concentrations were considered to be subtherapeutic in 40% and undetectable in 5% of samples. Supratherapeutic concentrations were reported for 39% of the samples assayed, 10% of which fell into a range considered to be toxic (>100 μg/ml).
Pharmacodynamic studies have demonstrated a strong relationship between 5-FC blood concentrations and both toxicity and efficacy. The strongest of these associations has been the toxicodynamic relationship of two adverse effects, bone marrow suppression and hepatic dysfunction. Several studies have shown that 5-FC concentrations greater than 100 μg/ml are toxic. The largest study that examined this relationship involved 194 patients with cryptococcal meningitis (83). The incidence of both bone marrow toxicity and hepatotoxicity was greater than 60% in patients with peak concentrations greater than 100 μg/ml (83). Conversely, these toxicities were observed in only 30% of patients with 5-FC concentrations below 100 μg/ml. Several other studies have confirmed this concentration-effect relationship (35, 50).
The pharmacodynamic investigation of 5-FC relative to treatment efficacy has been limited primarily to preclinical infection models (1, 44). Results from these models demonstrated a strong relationship among 5-FC concentrations in blood relative to the MIC and treatment efficacy. Maximal organism killing was observed at concentrations just above the MIC, and optimal outcomes were observed when blood concentrations exceeded the MIC for only 50% of the dosing interval (time above the MIC of 50%). A search for similar relationships in clinical trials has not been sought. However, if the MIC distribution for most Candida and cryptococcal isolates and the pharmacokinetics of 5-FC in patients are considered, it is possible that one could administer lower doses to achieve the pharmacodynamic target of a 50% time above the MIC.
Triazoles.
Four triazole compounds (fluconazole, itraconazole, voriconazole, and posaconazole) have been approved and are currently in wide use for the prevention and treatment of invasive fungal infections due to their relative safety, their broad-spectrum activity, and the availability of both parenteral (except posaconazole) and oral preparations. Despite similar mechanisms of action, structural differences among this group of antifungal drugs result in distinct pharmacokinetic properties for each compound, e.g., renal elimination for fluconazole and hepatic elimination for the other three compounds. A common characteristic of these compounds is the wide inter-and intraindividual variability of concentrations of drug in blood. The kinetic characteristics of three of these drugs (itraconazole, voriconazole, and posaconazole) meet the criteria suggesting the utility of therapeutic drug monitoring.
Fluconazole.
Fluconazole is available as an intravenous and oral formulation with an excellent bioavailability. Despite the variability of fluconazole pharmacokinetics, studies have not demonstrated a need for routine concentration monitoring (5, 16, 18, 21, 29, 78). However, in the presence of renal dysfunction or during renal replacement therapy (especially continuous hemofiltration), fluconazole exposure might be profoundly altered and difficult to predict based on the dosing regimen (11, 103). Preclinical and clinical studies have shown the association between drug dose, exposure, in vitro susceptibility, and response to therapy, suggesting that dosing may be critical for outcome in infections due to pathogens with decreased susceptibility (18, 63). Monitoring of fluconazole therapy might thus theoretically contribute to improving the response in selected patients such as those with renal dysfunction/renal replacement therapy or when decreased in vitro antifungal susceptibility has been documented.
Itraconazole.
Itraconazole is utilized in the therapy of a wide spectrum of invasive fungal infections including Candida, Aspergillus, endemic fungi, and dermatophytes. Three itraconazole formulations are available, allowing both parenteral and oral administration. Both pharmacokinetic studies of healthy volunteers and population pharmacokinetic investigations have identified wide interpatient kinetic variation (7, 16, 23, 41). The variation in blood concentrations is due largely to differences in absorption of the oral formulations. The degree of variation has also been shown to vary among formulations (17, 48, 54, 84, 96, 97). The most erratic absorption is observed with the capsule formulation. In general, the cyclodextrin formulation is more readily absorbed than the capsules, resulting in roughly a 30% larger area under the concentration curve (AUC) than that with the capsule preparation (84). The peak blood concentration at steady state following the intake of the oral solution at a dose of 200 mg every 12 h ranged from 0.513 to 2.278 μg/ml, with a median concentration of 1.326 μg/ml. In contrast, the peak blood concentration at steady state following administration of the capsule formulation at the same dose of 200 mg every 12 h ranged from 0.297 to 1.609 μg/ml, with a median value of 0.741 μg/ml (17). Factors that have an impact on the absorption of itraconazole include gastric pH and food and are also specific to the formulation (17, 48, 71, 72, 81, 93, 96). The absorption from capsules is pH dependent and requires an acidic environment for optimal absorption. Therefore, this formulation is optimally administered with a meal or low-pH beverage such as cola. Many drugs that are intended to increase gastric pH have a deleterious impact on the absorption of this itraconazole formulation (Table 2) (30, 40). In contrast, the absorption of the oral solution is enhanced when it is taken in a fasted state and is not impacted by gastric pH (84). Several patient factors, including mucosal disease associated with chemotherapy, have also been shown to impact itraconazole pharmacokinetic variability (23). Thus, it is not surprising that the variation in population kinetic studies is larger than that observed in studies with healthy volunteers (coefficients of variation of 83 to 115% compared to 47%, respectively) (23, 41). Studies including individuals with acute leukemia, bone marrow transplantation, and AIDS have consistently observed both variability and, in general, lower blood concentrations than those achieved with healthy volunteers (17, 23, 25, 26, 37, 38, 41, 75). For example, the median trough concentration in an immunosuppressed cohort receiving itraconazole capsules of 400 mg daily was only 0.31 μg/ml but ranged from undetectable to 0.8 μg/ml (38). Nearly 60% of patients did not achieve the defined target trough concentration of 0.5 μg/ml. The cyclodextrin solution formulation was examined in a similar manner. Median trough concentrations were more than twice those observed with the capsule formulation (0.66 μg/ml) (38). Still, nearly a quarter of patients did not achieve the defined target trough concentration of 0.5 μg/ml. In neutropenic cancer patients receiving a 7-day intravenous itraconazole regimen (250 mg/day) followed by 7-day therapy with the oral solution (400 mg/day), mean trough levels between 0.5 and 1.5 μg/ml were obtained (14). Another important pharmacokinetic variable shown to impact itraconazole exposure is drug interactions. Itraconazole, like all of the triazoles, exhibits multiple important drug interactions, most notably with cytochrome P450-inducing drugs (40).
TABLE 2.
Interacting drugs that may alter antifungal blood concentrations
| Drug | Interacting drug(s) that may decrease antifungal concn | Interacting drug(s) that may increase antifungal concn |
|---|---|---|
| Itraconazole | Antacidsa including aluminum carbonate (basic), aluminum hydroxide, aluminum phosphate, calcium, dihydroxyaluminum aminoacetate, dihydroxyaluminum sodium carbonate, magaldrate, magnesium carbonate, magnesium hydroxide, magnesium trisilicate, sodium bicarbonate; antibiotics including isoniazid, rifabutin, rifampin, rifapentine; antiepileptics including carbamazepine, fosphenytoin, phenytoin; antiretrovirals including darunavir, didanosine, efavirenz, etravirine, nevirapine; H2 blockersa including cimetidine, famotidine, nizatidine, ranitidine, roxatidine; barbituates including phenobarbital; protonpump inhibitorsa including esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole | Antibiotics including clarithromycin; antiretrovirals including amprenavir, darunavir, fosamprenavir, lopinavir, ritonavir |
| Voriconazole | Antibiotics including rifabutin, rifampin, rifapentine; antiepileptics including carbamazepine, fosphenytoin, phenytoin; antiretrovirals including amprenavir, darunavir, delavirdine, efavirenz, nevirapine, ritonavir, tipranavir; barbituates including alfuzosin, aprobarbital, butabarbital, eterobarb, heptabarbital, hexobarbital, mephobarbital, pentobarbital, phenobarbital, secobarbital | Antiretrovirals including delavirdine, etravirine, fosamprenavir, nelfinavir, nevirapine, saquinavir, tipranavir, efavirenz; oral contraceptives including ethinyl estradiol, norethindrone; proton pump inhibitors including omeprazole |
| Posaconazole | Antiepileptics including phenytoin; H2 blockers including cimetidine, famotidine, nizatidine, ranitidine, roxatidine; proton pump inhibitors including esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole; gastric motility agent including metoclopramide | Antiarrhythmics including quinidine; antibiotics including rifabutin; antiepileptics including phenytoin; antimalarials including halofantrine; antipsychotics including pimozide; antiretrovirals including etravirine; antihistamines including astemizole(off market), terfenadine (off market); benzodiazepines including midazolam; calcium channel blockers including amlodipine, diltiazem, felodipine, lercanidipine, nifedipine, nisoldipine, nitrendipine, verapamil; ergot alkaloids including dihydroergotamine, ergoloid mesylates, ergonovine, ergotamine, methylergonovine, methysergide; immunosuppressants including cyclosporine, sirolimus, tacrolimus; serotonic receptor antagonists including cisapride (off market); statins including atorvastatin, lovastatin, simvistatin; vinca alkaloids including vinblastine, vincristine, vincristine liposome, vinorelbine |
Impact the capsule formulation of itraconazole.
Numerous itraconazole concentration-effect studies have been undertaken, and each one has demonstrated a strong link to drug efficacy (10, 17, 25, 26, 72, 93). A similar relationship for toxicity has not been identified. The itraconazole pharmacodynamic efficacy investigations include both preclinical animal model study and clinical trials. The disease states studied include states induced by a wide variety of invasive fungal diseases including those due to Candida, Aspergillus, Cryptococcus, and Coccidioides. In addition, the utility of monitoring has been examined using two treatment strategies, including prophylaxis to prevent the development of an invasive fungal infection as well as treatment of documented infections. In a preclinical study of animals with invasive aspergillosis, investigators examined the relationship between itraconazole trough concentrations utilizing a microbiological assay and treatment effect. A maximal reduction in the burden of Aspergillus was observed in animals with trough concentrations greater than 6 μg/ml (10). These results correlate well with findings from Aspergillus treatment trials. For example, in a group of 21 patients with invasive aspergillosis, the mean itraconazole trough concentrations were 6.5 μg/ml for responders and only 4.2 μg/ml for nonresponders (based upon a microbiological assay) (25). A similar quantitative relationship was observed for a group of patients with coccidioidomycosis (nonmeningitis). Among this cohort of 39 patients, the concentration measured by bioassay was 6.5 μg/ml for the 28 patients who experienced a clinical response, while the concentration a cohort of 11 nonresponders was 4.0 μg/ml (93). In another study, investigators examined the impact of itraconazole trough concentrations (by HPLC) on treatment outcome for a group of 25 patients with human immunodeficiency virus and cryptococcal meningitis. Treatment success was observed for 100% of patients with trough concentrations exceeding 1 μg/ml. In contrast, only a partial clinical response was achieved for 66% of those patients with concentrations below 1 μg/ml (26). The largest database examining the relationship between itraconazole therapeutic drug monitoring and efficacy involved the therapy of oral mucosal candidiasis (78). The analysis included more than 250 patients from four treatment trials and examined the impact of both itraconazole trough concentration (by HPLC) and Candida in vitro susceptibility. The trough concentration associated with the highest treatment rate of success was 0.5 μg/ml, suggesting that lower exposures are needed the for the treatment of this organism and this localized infection site than for systemic fungal infection.
An additional clinical area of itraconazole monitoring has been in the study of antifungal drug prophylaxis for patients at high risk of invasive fungal infection (13, 37, 38, 89). These studies have also demonstrated a relationship between itraconazole blood concentration and effect. However, the itraconazole concentration associated with effective disease prevention is two-to fourfold lower than that shown to be necessary for fungal disease treatment. For example, two independent studies of itraconazole prophylaxis in neutropenic patients included assays of trough concentrations by HPLC (13, 89). Results from both investigations observed invasive fungal infection in 50% of the cohort with itraconazole concentrations of below 0.25 μg/ml. In contrast, only 30% of patients with concentrations exceeding this value presented with infections. In another study, the investigators demonstrated a relationship between itraconazole trough concentrations and patient mortality due to breakthrough invasive fungal infections (37). Among a group of 20 patients developing an invasive fungal infection while on prophylaxis, the median itraconazole trough concentration in those with fatal infections was 0.12 μg/ml, and those surviving the infection achieved a trough concentration of 0.69 μg/ml. Taken together, existing evidence supports therapeutic drug monitoring to optimize clinical efficacy when this drug is used for prophylaxis and therapy of invasive fungal infections.
Voriconazole.
Voriconazole is a more recently developed triazole antifungal with broad-spectrum antifungal activity, including enhanced potency against Aspergillus species (49). The compound is available in both intravenous and oral formulations. Studies with healthy volunteers demonstrated high oral bioavailability (96%) (55, 76, 77). While pharmacokinetic and pharmacodynamic studies of this compound are less complete than those with itraconazole, studies thus far demonstrate some of the cardinal indications for therapeutic drug monitoring.
Pharmacokinetic investigations with healthy volunteers demonstrated wide intersubject variability in blood concentrations (34, 76, 77). This variability was observed with both intravenous and oral formulations. Thus, unlike itraconazole, the variability was not associated primarily with absorption, at least in this healthy cohort. A nonlinear dose-exposure profile attributed to a saturation of hepatic metabolism of voriconazole has been observed for adult individuals. Subsequent studies demonstrated that most of the pharmacokinetic variability is due to differences in the ability to metabolize voriconazole via the CYP2C19 P450 enzyme (34, 45, 76, 77, 80). Polymorphisms in the gene encoding this enzyme are common and result in variable rates of voriconazole metabolism. Those patients who are homozygous extensive metabolizers at CYP2C19 have less than one-fourth and one-half the average blood concentrations of voriconazole compared to patients who are homozygous poor metabolizers or heterozygous extensive metabolizers at CYP2C19, respectively. While these polymorphisms can occur in any individual, they are more common in certain ethnic groups. For example, roughly 2 to 5% of Caucasians are homozygous poor metabolizers of CYP2C19, while 26 to 28% are heterozygous extensive metabolizers and 70 to 73% are homozygous extensive metabolizers. In contrast, patients of Asian descent have a 14 to 19% frequency of homozygous poor metabolizer status, 43 to 46% are heterozygous extensive metabolizers, and only 35 to 43% are homozygous extensive metabolizers (45, 46, 55, 76, 77, 80). Whether and how individual genotyping of CYP2C19 may be implemented in the clinical routine for predicting voriconazole exposure remain to be determined.
Additional factors that have been shown to impact voriconazole pharmacokinetics include liver disease, age, CYP2C19-and CYP3A-interacting comedications, and changes from intravenous to oral therapy (Table 2). While voriconazole is very well absorbed, for patients with above-average body weight, the change from a weight-based intravenous regimen of 4 mg/kg every 12 h to a fixed 200-mg dose twice daily will result in a marked dose reduction. For example, in a patient with a body weight of 75 kg, this regimen change would results in a 33% reduction in the amount of voriconazole administered.
Similar to studies with other drugs, population pharmacokinetic investigations have shown that interpatient variability is more common in certain patient cohorts (12, 27, 55, 59, 60, 65, 82, 90, 91, 92, 102). For example, in a recent study of bone marrow transplant recipients receiving voriconazole for antifungal prophylaxis, 87 patients had voriconazole trough concentrations assayed after 5 days of therapy. In this population, 15% had undetectable levels and 27% had concentrations below 0.5 μg/ml, while only 62% had measurements between 0.5 and 2 μg/ml (the median concentration in studies of healthy subjects) (91). These findings have been confirmed in a similar study of this patient population (92). Nearly 20% of patients in the second cohort were found to have trough concentrations more than fourfold lower than the median concentration in healthy volunteers. Investigation of voriconazole monitoring has also been undertaken in the solid-organ-transplant population (12). In a cohort of 48 lung transplant recipients, peak and trough concentrations were measured. Those investigators defined a therapeutic goal as trough concentrations of 1.5 μg/ml and peak values of 4 μg/ml. These concentration goals were met for only 20% of patients after 1 week. These observations were similar for patients receiving both oral and intravenous voriconazole therapy. In this patient group, doses of 800 mg per day were needed to achieve detectable concentrations. In a much smaller study of critically ill patients in an intensive care unit setting, eight patients were administered 200 mg voriconazole by nasogastric tube twice daily (59). The mean trough concentration in the small cohort was 4.6 μg/ml, but the variation was large. The trough concentrations for two of eight patients were less than 2 μg/ml, and the concentration was undetectable in one patient. Moreover, a decline in blood concentrations during voriconazole therapy lasting more than 2 months has been reported (60).
In contrast to adult patients, a linear nonsaturable dose-exposure pharmacokinetic profile has been described for children (101). In order to obtain efficacious blood levels, daily doses of up to 14 mg/kg are needed in the pediatric setting (compared with 6 to 8 mg/kg for adults). However, blood concentration variability and subtherapeutic values in the pediatric population have also been reported (68). These population pharmacokinetic data suggest that multiple variables other than CYP2C19 metabolizer status may have an impact on voriconazole drug exposure.
An accumulating number of investigations have examined the pharmacodynamic relationship between voriconazole exposure and treatment efficacy. Preclinical animal model studies have defined the voriconazole exposure associated with treatment efficacy against Candida species. Using an invasive candidiasis model, studies demonstrated that a voriconazole exposure expressed as the unbound AUC in relation to the MIC needed to produce 50% of the maximal microbiological effect or a 2-log reduction in organism burden compared to controls was a free-drug value near 25 (2). The value identified for this triazole is nearly identical to that shown to be of importance for other drugs in this class, including fluconazole and posaconazole (2, 3, 5).
Examination of clinical candidemia trial data suggests that a similar relationship is relevant for patient outcome (18, 70, 78). While therapeutic drug monitoring was not performed in these trials, organism MIC data and drug dose are available. Using estimates of patient AUC, one can examine the relationship between the voriconazole free-drug AUC/MIC ratio and patient outcome. In the patient cohort with estimated free-drug AUC/MIC ratios of below 25, clinical success ranged from 52 to 60%. However, for the group with estimated free-drug AUC/MIC exposures of 32 or greater, success was reported for nearly 80% of patients. Several studies also examined the impact of voriconazole exposure on the treatment of another invasive fungal infection, aspergillosis. The FDA briefing document for voriconazole reported a pharmacokinetic and pharmacodynamic analysis of 280 patients with proven or probable invasive fungal infections, suggesting a trend of higher success rates (56% success with values of >0.5 μg/ml, compared to 46% success with values below 0.5 μg/ml; odds ratio, 1.5; 95% confidence interval 0.6 to 3.4) with mean voriconazole concentrations above 0.5 μg/ml (34). The majority of these pharmacokinetic estimates is based upon single trough concentrations. It would be interesting to determine if the use of additional monitoring time points to more accurately reflect the AUC will further enhance the strength of these concentration-outcome relationships.
Another large data set examined random voriconazole concentrations for 142 patients from an open-label treatment trial of aspergillosis (27). Similar to other voriconazole pharmacokinetic studies, a wide range of concentrations was observed (range, <0.1 μg/ml to 9.7 μg/ml). The patient cohort with concentrations less than 0.25 μg/ml experienced treatment failure 80% of the time, while those with values equal to or above 0.5 μg/ml were treated successfully in nearly 70% of cases.
An additional clinical voriconazole exposure-response analysis was undertaken in a retrospective study of 188 patients with invasive fungal infections. Twenty-eight patients in this cohort had concentrations measured because of perceived disease progression (n = 17) or hepatotoxicity (n = 11) (82). Twenty-four of the 28 patients had probable or proven invasive aspergillosis. Ten patients with concentrations greater than 2.0 μg/ml survived. Forty-four percent of patients with a concentration of less than 2.0 μg/ml survived (P < 0.02). The voriconazole dose was increased for 11 patients with concentrations of less than 2.0 μg/ml, 8 of whom survived. In a recent prospective investigation of voriconazole serum concentration monitoring for patients with invasive fungal infections, treatment outcome was also statistically linked to drug concentration (65). More than 180 voriconazole concentrations were measured for 52 patients over more than 2,000 treatment days. Patients with voriconazole drug concentrations of less than 1 μg/ml exhibited a response rate of just over 50%. Conversely, patients with concentrations over 1 μg/ml experienced a response rate of 90%. Taken together, these studies suggest a relationship between voriconazole exposure and treatment efficacy. Whether the optimal concentration of voriconazole is a trough concentration of 0.5, 1.0, or 2.0 μg/ml remains to be defined in future studies.
A prospective investigation of the impact of voriconazole therapeutic drug monitoring in the setting of antifungal prophylaxis has also been undertaken. Among a group of patients receiving voriconazole prophylaxis following allogeneic hematopoietic stem cell transplantation, 43 underwent trough blood monitoring. A group of six patients developed breakthrough candidiasis. The mean trough blood concentration in this group was less than 0.5 μg/ml, compared to a mean trough concentration of greater than 2 μg/ml in the remaining cohort (P = 0.061) (91). While that report involved a small number of patients, the data suggest that the voriconazole concentration necessary to prevent a fungal infection may be lower than that needed to treat an established infection. This relationship is similar to that observed with itraconazole.
Other clinical investigations in the area of toxicodynamics have been reported (34, 74). In the clinical development program, two interesting associations between voriconazole pharmacokinetics and toxicity were observed. The most common adverse effect associated with the administration of this compound is a self-limited visual phenomenon termed photopsia. The median voriconazole blood concentration for patients reporting this side effect in clinical trials was 3.52 μg/ml; the median concentration for those without this visual symptom was 2.72 μg/ml. Because of the self-limited and fully reversible nature of this phenomenon, this relationship should not warrant therapeutic drug monitoring (15).
Another potentially more significant but much less common toxicity associated with the administration of all triazole drugs, including voriconazole, is hepatotoxicity (15, 24, 58, 65, 87). A pharmacodynamic evaluation of voriconazole and liver function abnormalities was undertaken, and a relationship was identified. Studies demonstrated that the risk of elevation of a liver function laboratory value increased by 7 to 17% for every 1-μg/ml increase in the random voriconazole concentration (58). The relative risk of this toxic effect is low, and it has been argued that monitoring based on this association is not warranted. However, case reports of severe liver dysfunction due to unusually high voriconazole concentrations have surfaced in the literature (79).
Most recently, reversible neurological symptoms in patients with elevated voriconazole concentrations have been described (15, 47, 65, 69). Central and peripheral neurological toxicities have been reported for patients with trough concentrations ranging from three to five times the normal median values (5.5 to 14 μg/ml). A recent prospective investigation of voriconazole serum concentrations in a cohort of 52 patients receiving drug for the treatment of a variety of invasive fungal infections identified four patients with encephalopathy (confusion, hallucinations, and myoclonia), which was attributed to elevated voriconazole concentrations (65). In each case, the voriconazole value was greater than 5.5 μg/ml and was associated with the concomitant use of the 2C19 inhibitor omeprazole. The side effects resolved completely within 1 to 3 days after the discontinuation of the drug in each case. The probability of neurological symptoms in the small group of patients with trough concentrations of 8 μg/ml was 90%. Conversely, no neurological symptoms were observed in the group of 48 patients with trough concentrations below 5.5 μg/ml. Given the self-limited nature of the visual side effect and the infrequency and reversibility of the associated liver function abnormalities, many clinicians have not pursued monitoring on the basis of toxicity. However, signs of encephalopathy occurring during voriconazole therapy should prompt blood level measurements and drug discontinuation. Routine monitoring of blood levels during the first 7 days of therapy may contribute to the early recognition of drug accumulation and the prevention of this adverse event.
Posaconazole.
Posaconazole is the most recently approved triazole antifungal (61, 62). The drug exhibits an enhanced spectrum against filamentous fungi, including the emerging zygomycete fungal group. Posaconazole is available as an oral formulation and has a long elimination half-life (>24 h). Despite the prolonged half-life, the compound is optimally administered multiple times daily (two to four times) due to saturable absorption. Similar to the triazoles voriconazole and itraconazole, pharmacokinetic investigation has identified marked interpatient variability for both healthy volunteers and patient populations (19, 20, 39, 51, 52, 56, 94).
A number of factors have been demonstrated to impact posaconazole absorption including food (and fat specifically), gastric pH (and the use of proton pump inhibitors), mucosal health, and frequency of administration (due to saturable absorption) (53). Clinical pharmacokinetic studies have shown that the coadministration of posaconazole with a nutritional supplement containing 14 g of fat increases the AUC by more than 200% (51). Administration with a high-fat meal has been shown to enhance absorption by nearly fourfold. The impact of gastric pH on the absorption of posaconazole appears to be similar to the relationship described above for itraconazole capsules. The coadministration of omeprazole was found to reduce the serum Cmax and AUC by 50% and 30%, respectively (Table 2). Conversely, the administration of posaconazole with a low-pH beverage enhanced absorption and increased the Cmax and AUC by 90% and 70%, respectively. Another medication shown to adversely impact posaconazole absorption (reduce the AUC by 20%) is metoclopramide, presumably due to a reduced mucosal transit time.
Saturable absorption has also been shown to markedly impact posaconazole bioavailability. In a study with healthy volunteers, the fractionation of an 800-mg/day dosage into a dose of 400 mg twice daily was shown to increase the serum AUC by nearly 100%; further fractionation of the regimen to 200 mg four times daily enhances bioavailability by 220% (33).
Not surprisingly, pharmacokinetic variability has been more pronounced in cohorts of ill patients. The groups examined in the population pharmacokinetic investigations would be expected to have gastric mucosal alteration and reduced food intake due to mucositis. For example, in one study of 98 patients with refractory febrile neutropenia or known invasive fungal infection, concentrations of posaconazole were 52% lower in allogeneic bone marrow transplant recipients than in patients without transplants (39). In that study, the coefficient of variation was large (71 to 82%). A similar pharmacokinetic unpredictability was observed in a study of a population of neutropenic stem cell transplant recipients (variability of 38 to 68%) (94). The largest experience with posaconazole serum concentration monitoring has come from clinical monitoring experience at Mira Vista laboratories. Among the monitoring results from more than 800 patients, concentrations have ranged from undetectable in 3% to greater than 10 μg/ml in 4% of patients (Joe Wheat, Mira Vista, personal communication).
The relationship between exposure and efficacy has been examined in both preclinical animal infection models and two clinical trials. Similar to the other triazoles, the pharmacodynamic exposure associated with efficacy in a murine candidiasis model identified a free-drug 24-h AUC/MIC target ratio of 20 to 25 (3). Animal model pharmacodynamic target investigation with other fungal organisms has not been undertaken.
Limited clinical pharmacokinetic and pharmacodynamic data are available. However, the single published posaconazole clinical treatment trial that examined the impact of exposure on treatment outcome was in the setting of an open-label investigation for the treatment of refractory invasive aspergillosis (99). Among this group of 67 patients, the clinical response correlated with the blood posaconazole concentration over a wide range of values. The patients in the lowest concentration quartile achieved an average blood concentration of 0.13 μg/ml or less and experienced the lowest rate of clinical response (20%). In the group with the highest clinical response (70%), the average steady-state concentration of posaconazole was 1.25 μg/ml (the highest concentration quartile). Patients with concentrations in the middle two quartiles (average concentrations, 0.5 to 0.7 μg/ml) were successfully treated 53% of the time.
A second investigation examined the relationship between concentrations of posaconazole in blood and efficacy in a prophylaxis trial of patients undergoing allogeneic hematopoietic stem cell transplantation and graft-versus-host disease (95). The posaconazole regimen prevented invasive fungal infection in 97.6% of patients and was superior to fluconazole. However, posaconazole concentrations were nearly twofold lower in the small group of patients (n = 5) who developed an invasive fungal infection than in the cohort (n = 241) that did not develop infection (C average of 0.611 μg/ml in the infected cohort and C average of 0.922 μg/ml in the uninfected cohort, respectively). These differences were not statistically significant, possibly due to the small number of patients who developed infection (2.4%). While these studies are less extensive than those available for the other antifungals, a relationship between exposure and efficacy is suggested for this compound, with marked kinetic variability. Thus, the concept of posaconazole therapeutic drug monitoring should be explored in future clinical studies.
POLYENES AND ECHINOCANDINS
Clear pharmacodynamic relationships for drugs from the polyene and echinocandin drug classes have been elucidated: the pharmacodynamic indices peak blood level and AUC over MIC have been recognized as the best predictors of treatment response (4, 6). However, pharmacokinetic studies with drugs from these antifungal drug classes are either lacking or do not meet the criteria for therapeutic drug monitoring. The limited population pharmacokinetic data available demonstrate predictable inter-and intraindividual dose-exposure relationships for these antifungals (8, 31, 42, 43, 85, 100). The greatest degree of variability in these investigations was due to extremes of patient weight. A recent report described low caspofungin concentrations in patients hospitalized in intensive care units compared with concentrations in patients hospitalized in wards. The clinical significance of this observed pharmacokinetic variation is unknown (66). The role of monitoring of therapy with echinocandins in selected clinical settings has not been explored in prospective clinical studies.
SUMMARY AND FUTURE DIRECTIONS
Unpredictable interindividual pharmacokinetic variability by a factor 50 to 100 has been demonstrated for 5-FC and three triazole antifungals. Validated chromatographic and/or microbiological drug assays have been developed for each of these compounds. However, at present, these assays are available at only a few reference laboratories, which makes the routine application of real-time monitoring of antifungal therapy difficult. Several clinical trials suggested that therapeutic drug monitoring of 5-FC and azoles antifungals can be useful to both reduce drug toxicity and optimize efficacy. However, prospective controlled studies rigorously establishing the drug levels associated with treatment response or toxicity or showing that drug level monitoring with dose adjustment based upon drug level improves clinical outcome have not been conducted.
Evidence for and against the monitoring of antifungal blood levels are summarized in Table 3. The proposed target concentrations in this review are based upon limited data suggesting an association between blood level and response or toxicity and are thus provided as tentative guidelines and not an established standard of care.
TABLE 3.
Evidence for and against monitoring of blood levels during azole therapy
| Evidence for monitoring of blood levels during azole therapy | Evidence against monitoring of blood levels during azole therapy |
|---|---|
| Large and unpredictable variability of blood levels | Real-time measurements not routinely available |
| Multiple factors influencing drug absorption, distribution, and elimination, including age, genetic background, compliance and gastrointestinal function, comedication, and liver and/or renal dysfunction | Target blood levels not established; lacking data from prospective controlled studies systematically exploring efficacy and toxicity associated with drug over-or underdosing |
| Emergence of fungal pathogens with decreased susceptibility requiring optimal adjustment of drug exposure | Drug blood concn might not reflect exposure and efficacy in infected tissues |
| Multiple clinical reports of failure associated with drug underdosing and toxicity associated with drug overdosing |
Until additional prospective evaluations provide answers to these questions, it seems reasonable to offer the following guidance for those wishing to incorporate this tool into patient management (Table 4). For 5-FC, peak concentration monitoring should be considered for all patients early in the course of therapy (3 to 5 days) to reduce the incidence of toxicity. Measurements should also be performed when there are clinical manifestations of toxicity or any change in renal function. The target 5-FC peak concentration range should be 20 to 50 μg/ml.
TABLE 4.
Tentative recommendations for monitoring of blood levels during antifungal therapy
| Drug | Indication | Time of first measurement after start of therapy (days) | Target blood concna (μg/ml) for:
|
|
|---|---|---|---|---|
| Efficacy | Safety | |||
| Flucytosine | Routine during first wk of therapy, renal insufficiency, lacking response to therapy | 3-5 | Peak of >20 | Peak of <50 |
| Itraconazole | Routine during first wk of therapy, lacking response, gastrointestinal dysfunction, comedication | 4-7 | For prophylaxis, trough of >0.5; for therapy, trough of >1 to 2 | NA |
| Voriconazole | Lacking response; gastrointestinal dysfunction; comedication; children; intravenous-to-oral switch; severe hepatopathy; unexplained neurological symptoms/signs | 4-7 | For prophylaxis, trough of >0.5; for therapy, trough of >1 to 2 | Trough of <6 |
| Posaconazole | Lacking response; gastrointestinal dysfunction, therapy with proton pump inhibitors; comedication | 4-7 | For prophylaxis, trough of >0.5; for therapy, trough of >0.5 to 1.5 | NA |
Total or bound and unbound drug concentrations. NA, not applicable.
For itraconazole, trough concentrations should be assayed for all patients early in the course of prophylaxis or therapy (4 to 7 days), with evidence of clinical failure, or following the initiation of any drug demonstrated to alter itraconazole metabolism. Trough concentration goals for prophylaxis using an HPLC assay should be 0.5 μg/ml or greater and should be 1 to 2 μg/ml in the case of treatment of fungal infection.
Monitoring of voriconazole trough concentrations may be considered early in therapy (4 to 7 days) for all patients to determine if measurable or excess concentrations are present. The assay should also be considered for patients with poor clinical response, the addition of an interacting medication, a change in the route of administration of voriconazole (e.g., switch from a mg/kg dosing schedule for intravenous therapy to a fixed dosing schedule for oral therapy), deteriorating hepatic function, and suspected toxicity such as severe hepatic dysfunction or neurological signs consistent with drug overdosing. A definitive voriconazole trough concentration goal has not yet been identified but is likely to be in the range of 0.5 to 2.0 μg/ml for efficacy and less than 6.0 μg/ml for toxicity.
For posaconazole, trough concentration monitoring may be considered for all patients early in therapy (4 to 7 days). Similar to the other triazoles, an assay should also be considered if there is poor clinical efficacy, after the addition of an interacting medication, if there is a change in the dosing regimen, or if there are conditions that put a patient at risk of impaired gastrointestinal absorption (e.g., severe mucositis, vomiting, diarrhea, ileus, graft-versus-host disease, impaired dietary intake, and therapy with proton pump inhibitors). The trough posaconazole goal should be 0.5 to 1.5 μg/ml for patients being treated for invasive fungal infection. The goal for prophylaxis is not clear but may be lower as described above for the related antifungal itraconazole, i.e., more than 0.5 μg/ml. However, saturable oral absorption might be a limiting factor for obtaining efficacious blood levels after dose adjustment. The development of an intravenous formulation in the future may contribute to more easily achieving these target concentrations.
Despite a high degree of variability in the levels of fluconazole in blood, especially in patients with renal failure or during renal replacement therapy (continuous hemofiltration), and the established relationship between drug exposure, in vitro antifungal susceptibility, and efficacy, no specific target blood levels have been identified in clinical studies, and no specific recommendations for monitoring can be formulated.
Similarly, data available at present do not allow the formulation of recommendations for monitoring of blood levels during therapy with polyenes and echinocandins.
Further studies are desperately needed to identify the optimal timing of monitoring, to refine the concentration goals, and to better delineate targeted monitoring for specific patient populations and clinical scenarios. Examples of common and important clinical scenarios include an identification of concentration target goals for combination antifungal therapy and treatment of pharmacologically privileged sites (e.g., the central nervous system).
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Andes, D., and M. van Ogtrop. 2000. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine disseminated candidiasis model. Antimicrob. Agents Chemother. 44:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., K. Marchillo, T. Stamstad, et al. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., K. Marchillo, R. Conklin, et al. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D. 2001. In vivo pharmacodynamics of amphotericin B against selected Candida species. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes, D., and M. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes, D., D. J. Diekema, M. A. Pfaller, R. A. Prince, K. Marchillo, J. Ashbeck, and J. Hou. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Enhanced bioavailability of itraconazole in hydroxypropyl-β-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob. Agents Chemother. 42:1862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett, J. E., W. E. Dismukes, R. J. Duma, et al. 1979. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N. Engl. J. Med. 301:126-131. [DOI] [PubMed] [Google Scholar]
- 10.Berenguer, J., N. M. Ali, C. M. Allende, et al. 1994. Itraconazole for experimental pulmonary aspergillosis: comparison with amphotericin B, interaction with cyclosporin A, and correlation between therapeutic response and itraconazole concentrations in plasma. Antimicrob. Agents Chemother. 38:1303-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berl, T., K. D. Wilner, M. Gardner, R. A. Hansen, B. Farmer, B. A. Baris, and W. L. Henrich. 1995. Pharmacokinetics of fluconazole in renal failure. J. Am. Soc. Nephrol. 6:242-247. [DOI] [PubMed] [Google Scholar]
- 12.Billaud, E. M., et al. 2006. Voriconazole therapeutic drug monitoring (TDM) in cystic fibrosis lung transplanted patients, abstr. O-5-2006. Abstr. 16th Congr. Int. Soc. Hum. Anim. Mycol., 25 to 29 June 2006, Paris, France.
- 13.Boogaerts, M. A., G. E. Verhoef, P. Zachee, et al. 1989. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 32:103-108. [DOI] [PubMed] [Google Scholar]
- 14.Boogaerts, M. A., J. Maertens, R. Van Der Geest, A. Bosly, J. M. Michaux, A. Van Hoof, M. Cleeren, R. Wostenborghs, and K. De Beule. 2001. Pharmacokinetics and safety of a 7-day administration of intravenous itraconazole followed by a 14-day administration of itraconazole oral solution in patients with hematologic malignancy. Antimicrob. Agents Chemother. 45:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd, A. E., S. Modi, C. B. Howard, S. J. Moore, B. G. Keevil, and D. E. Denning. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. [DOI] [PubMed] [Google Scholar]
- 16.Brammer, K. W., P. R. Farrow, and J. K. Faulkner. 1990. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev. Infect. Dis. 12(Suppl. 3):318-326. [DOI] [PubMed] [Google Scholar]
- 17.Cartledge, J. D., J. Midgely, and B. G. Gazzard. 1997. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J. Clin. Pathol. 50:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clancy, C. J., V. L. Yu, A. J. Morris, et al. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courtney, R., D. Wexler, E. Radwanski, et al. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtney, R., A. Sansone, A. Calzetta, M. Marinho, and M. Laughlin. 2003. The effect of a nutritional supplement (Boost Plus) on the oral bioavailability of posaconazole, abstr. M-1604. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., 14 to 17 September 2003, Chicago, IL.
- 21.Csajka, C., L. A. Décosterd, T. Buclin, J. L. Pagani, K. Fattinger, J. Bille, and J. Biollaz. 2001. Population pharmacokinetics of fluconazole given for secondary prevention of oropharyngeal candidiasis in HIV-positive patients. Eur. J. Clin. Pharmacol. 57:723-727. [DOI] [PubMed] [Google Scholar]
- 22.Cutler, R. E., A. D. Blair, and M. R. Kelly. 1978. Flucytosine kinetics in subjects with normal and impaired renal function. Clin. Pharmacol. Therapeut. 24:333-342. [DOI] [PubMed] [Google Scholar]
- 23.de la Vega, L., S. P. Volkow, R. A. Yeates, et al. 1994. Administration of the antimycotic agents fluconazole and itraconazole to leukaemia patients: a comparative pharmacokinetic study. Drugs Exp. Clin. Res. 20:69-75. [PubMed] [Google Scholar]
- 24.den Hollander, J. G., C. van Arkel, B. J. Rijnders, P. J. Lugtenburg, S. de Marie, and M. D. Levin. 2006. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J. Antimicrob. Chemother. 57:1248-1250. [DOI] [PubMed] [Google Scholar]
- 25.Denning, D. W., R. M. Tucker, L. Hanson, et al. 1989. Treatment of invasive aspergillosis with itraconazole. Am. J. Med. 86:791-800. [DOI] [PubMed] [Google Scholar]
- 26.Denning, D. W., R. M. Tucker, L. H. Hanson, et al. 1989. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch. Intern. Med. 149:2301-2308. [PubMed] [Google Scholar]
- 27.Denning, D. W., P. Ribaud, N. Milpied, et al. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 28.Dismukes, W. E., G. Cloud, H. A. Gallis, et al. 1987. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared to six weeks. N. Engl. J. Med. 317:334-341. [DOI] [PubMed] [Google Scholar]
- 29.Dodds, L., R. Lewis, J. S. Lewis, C. Martin, and D. Andes. 2006. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 43(Suppl. 1):28-39. [Google Scholar]
- 30.Dominguez-Gill, H. A., N. A. Sanchez, and M. J. Garcia Sanchez. 2006. Therapeutic drug monitoring of itraconazole and the relevance of pharmacokinetic interactions. Clin. Microbiol. Infect. 12(Suppl. 7):97-106.16460557 [Google Scholar]
- 31.Dowell, J. A., W. Knebel, T. Ludden, M. Stogniew, D. Krause, and T. Henkel. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590-598. [DOI] [PubMed] [Google Scholar]
- 32.Drusano, G. L. 2004. How does a patient maximally benefit from anti-infective chemotherapy? Clin. Infect. Dis. 39:1245-1246. [DOI] [PubMed] [Google Scholar]
- 33.Ezzet, F., D. Wexler, R. Courtney, et al. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. 2001. Briefing document for voriconazole. Food and Drug Administration, Washington, DC. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3792b2.htm.
- 35.Francis, P., and T. J. Walsh. 1992. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin. Infect. Dis. 15:1003-1018. [DOI] [PubMed] [Google Scholar]
- 36.Gerzenshtein, L., S. M. Patel, K. K. Scarsi, M. J. Postelnick, and J. P. Flaherty. 2005. Breakthrough Candida infections in patients receiving voriconazole. Ann. Pharmacother. 39:1342-1345. [DOI] [PubMed] [Google Scholar]
- 37.Glasmacher, A., C. Hahn, C. Leutner, et al. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443-451. [DOI] [PubMed] [Google Scholar]
- 38.Glasmacher, A., C. Hahn, E. Molitor, et al. 1999. Itraconazole trough concentrations in antifungal prophylaxis with six different dosing regimens using hydroxylpropyl-beta-cyclodextrin oral solution or coated pellet capsules. Mycoses 42:591-600. [DOI] [PubMed] [Google Scholar]
- 39.Gubbins, P. O., G. Krishna, A. Sansone-Parsons, et al. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gubbins, P. O., and J. R. Amsden. 2005. Drug-drug interactions of antifungal agents and implications for patient care. Expert Opin. Pharmacother. 6:2231-2243. [DOI] [PubMed] [Google Scholar]
- 41.Hardin, T. C., J. R. Graybill, R. Fetchick, et al. 1988. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob. Agents Chemother. 32:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hebert, M. F., H. E. Smith, T. C. Marbury, S. K. Swan, W. B. Smith, R. W. Townsend, D. Buell, J. Keirns, and I. Bekersky. 2007. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J. Clin. Pharmacol. 45:1145-1152. [DOI] [PubMed] [Google Scholar]
- 43.Hong, Y., P. J. Shaw, C. E. Nath, S. P. Yadav, K. R. Stephen, J. W. Earl, and A. J. McLachlan. 2006. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob. Agents Chemother. 50:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hope, W. W., P. A. Warn, A. Sharp, et al. 2006. Derivation of an in vitro drug exposure breakpoint for flucytosine against Candida albicans and the impact of the MIC, growth rate, and resistance genotype on the antifungal effect. Antimicrob. Agents Chemother. 50:3680-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda, Y., K. Umenura, K. Kondo, K. Sekiguchi, S. Miyoshi, and M. Makashima. 2004. Pharmacokinetics of voriconazole and cytochrome P4509 2C19 genetic status. Clin. Pharmacol. Ther. 75:587-588. [DOI] [PubMed] [Google Scholar]
- 47.Imhof, A., D. J. Schaer, U. Schanz, and U. Schwarz. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739-742. [DOI] [PubMed] [Google Scholar]
- 48.Jaruratanasirikul, S., and A. Kleepkaew. 1997. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur. J. Clin. Pharmacol. 52:235-237. [DOI] [PubMed] [Google Scholar]
- 49.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 50.Kauffman, C. A., and P. T. Frame. 1977. Bone marrow toxicity associated with 5-fluorocytosine therapy. Antimicrob. Agents Chemother. 11:244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishna, G., M. Martinho, P. Chandrasekar, A. J. Ullmann, and H. Patino. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627-1636. [DOI] [PubMed] [Google Scholar]
- 52.Krishna, G., A. Sansone-Parsons, M. Martinho, B. Kantesaria, and L. Pedicone. 2007. Posaconazole plasma concentrations in juvenile patients with invasive fungal infection Antimicrob. Agents Chemother. 51:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishna, G., A. Moton, L. Ma, et al. 2008. Effect of gastric pH, dosing regimen and prandial state, food and meal timing relative to dose, and gastrointestinal motility on absorption and pharmacokinetics of the antifungal, posaconazole, abstr. P1264. Abstr. 18th Eur. Congr. Clin. Microbiol. Infect. Dis.
- 54.Lange, D., J. H. Pavao, J. Wu, and M. Klausner. 1997. Effect of a cola beverage on the bioavailability of itraconazole in the presence of H2 blockers. J. Clin. Pharmacol. 37:535-540. [DOI] [PubMed] [Google Scholar]
- 55.Lazarus, H. M., J. L. Blumer, S. Yanovich, et al. 2002. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J. Clin. Pharmacol. 42:395-402. [PubMed] [Google Scholar]
- 56.Lewis, R., H. Hogan, H. Howell, and A. Safdar. 2008. Progressive fusariosis: unpredictable posaconazole bioavailability, and feasibility of recombinant interferon-gamma plus granulocyte macrophage-colony stimulating factor for refractory disseminated infection. Leuk. Lymphoma 49:163-165. [DOI] [PubMed] [Google Scholar]
- 57.Lin, J. S., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 58.Lutsar, I., M. R. Hodges, K. Tomaszeewski, et al. 2003. Safety of voriconazole and dose individualization. Clin. Infect. Dis. 36:1087. [DOI] [PubMed] [Google Scholar]
- 59.Mohammedi, I., M. A. Piens, C. Padoin, et al. 2005. Plasma levels of voriconazole administered via a nasogastric tube to critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 24:358-360. [DOI] [PubMed] [Google Scholar]
- 60.Mulanovich, V., R. E. Lewis, I. A. Raad, and D. P. Kontoyiannis. 2007. Random plasma concentrations of voriconazole decline over time. J. Infect. 55:129-130. [DOI] [PubMed] [Google Scholar]
- 61.Muller, C., M. Arndt, C. Queckenberg, et al. 2006. HPLC analysis of the antifungal agent posaconazole in patients with haematological diseases. Mycoses 49:17-22. [DOI] [PubMed] [Google Scholar]
- 62.Nagappan, V., and S. Deresinki. 2007. Posaconazole: a broad spectrum triazole antifungal agent. Clin. Infect. Dis. 15:1610-1617. [DOI] [PubMed] [Google Scholar]
- 63.Pai, M. P., R. S. Turpin, and K. W. Garey. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pascual, A., V. Nieth, T. Calandra, J. Bille, S. Bolay, L. A. Decosterd, et al. 2007. Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob. Agents Chemother. 51:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 66.Pascual, A. A., L. Senn, S. Bolay, B. Rochat, P. Eggimann, R. Ksontini, T. Buclin, Bille J., T. Calandra, and O. Marchetti. 2007. Low total plasma concentration of caspofungin in surgical intensive care unit patients, abstr. M2025, p.465. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 67.Pasqualotto, A. C., S. J. Howard, C. B. Moore, et al. 2007. Flucytosine therapeutic monitoring: 15 years experience from the UK. J. Antimicrob. Chemother. 9:791-793. [DOI] [PubMed] [Google Scholar]
- 68.Pasqualotto, A. C., M. Shah, R. Wynn, and D. W. Denning. 2008. Voriconazole plasma monitoring. Arch. Dis. Child. 93:578-581. [DOI] [PubMed] [Google Scholar]
- 69.Pennick, G. J., M. Clark, D. A. Sutton, and M. G. Rinaldi. 2003. Development and validation of a high-performance liquid chromatography assay for voriconazole. Antimicrob. Agents Chemother. 47:2348-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, et al. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poirier, J. M., F. Berlioz, F. Isnard, et al. 1996. Marked intra-and inter-patient variability of itraconazole steady state plasma concentrations. Therapie 51:163-167. [PubMed] [Google Scholar]
- 72.Poirier, J. M., and G. Cheymol. 1998. Optimisation of itraconazole therapy using target drug concentrations. Clin. Pharmacokinet. 35:461-473. [DOI] [PubMed] [Google Scholar]
- 73.Polak, A., and H. J. Scholer. 1975. Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy 21:113-130. [DOI] [PubMed] [Google Scholar]
- 74.Potoski, B. A., and J. Brown. 2002. The safety of voriconazole. Clin. Infect. Dis. 35:1273-1275. [DOI] [PubMed] [Google Scholar]
- 75.Prentice, A. G., D. W. Warnock, S. A. Johnson, M. J. Phillips, and D. A. Oliver. 1994. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J. Antimicrob. Chemother. 34:247-252. [DOI] [PubMed] [Google Scholar]
- 76.Purkins, L., N. Wood, K. Greenhalgh, et al. 2003. Voriconazole, a novel wide spectrum triazole: oral pharmacokinetics and safety. Br. J. Clin. Pharmacol. 56(Suppl. 1):10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Purkins, L., N. Wood, P. Ghahramani, et al. 2002. Pharmacokinetics and safety of voriconazole following intravenous-to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rex, J. H., M. A. Pfaller, J. N. Galgiani, et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 79.Scherpbier, H. J., M. I. Hilhorst, and T. W. Kuijpers. 2003. Liver failure in a child receiving highly active antiretroviral therapy and voriconazole. Clin. Infect. Dis. 37:828-830. [DOI] [PubMed] [Google Scholar]
- 80.Service, R. F. 2005. Going from genome to pill. Science 308:1858-1860. [DOI] [PubMed] [Google Scholar]
- 81.Sharkey, P. K., M. G. Rinaldi, J. F. Dunn, T. C. Hardin, R. J. Fetchick, and J. R. Graybill. 1991. High-dose itraconazole in the treatment of severe mycoses. Antimicrob. Agents Chemother. 35:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith, J., N. Safdar, V. Knasinski, et al. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stamm, A. M., R. Diasio, W. E. Dismukes, et al. 1987. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am. J. Med. 83:236-242. [DOI] [PubMed] [Google Scholar]
- 84.Stevens, D. A. 1999. Itraconazole in cyclodextrin solution. Pharmacotherapy 19:603-611. [DOI] [PubMed] [Google Scholar]
- 85.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single-and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Summers, K. K., T. C. Hardin, S. J. Gore, and J. R. Graybill. 1997. Therapeutic drug monitoring of systemic antifungal therapy. J. Antimicrob. Chemother. 40:753-764. [DOI] [PubMed] [Google Scholar]
- 87.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 88.Touw, D. J., C. Neef, A. H. Thomson, and A. A. Vinks. 2005. Cost-effectiveness of therapeutic drug monitoring: a systematic review. Ther. Drug Monit. 27:10-17. [DOI] [PubMed] [Google Scholar]
- 89.Tricot, G., E. Joosten, M. A. Boogaerts, et al. 1987. Ketoconazole vs. itraconazole for antifungal prophylaxis in patients with severe granulocytopenia: preliminary results of two nonrandomized studies. Rev. Infect. Dis. 9(Suppl. 1):S94-S99. [DOI] [PubMed] [Google Scholar]
- 90.Trifilio, S., R. Ortiz, G. Pennick, A. Verma, J. Pi, V. Stosor, et al. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509-513. [DOI] [PubMed] [Google Scholar]
- 91.Trifilio, S., G. Pennick, J. Pi, J. Zook, M. Golf, K. Kaniecki, et al. 2007. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 109:1532-1535. [DOI] [PubMed] [Google Scholar]
- 92.Trifilio, S. M., C. L. Bennett, P. R. Yarnold, et al. 2007. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 39:425-429. [DOI] [PubMed] [Google Scholar]
- 93.Tucker, R. M., D. W. Denning, E. G. Arathoon, et al. 1990. Itraconazole therapy for nonmeningeal coccidioidomycosis: clinical and laboratory observations. J. Am. Acad. Dermatol. 23:593-601. [DOI] [PubMed] [Google Scholar]
- 94.Ullmann, A. J., O. A. Cornely, A. Burchardt, et al. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ullmann, A. J., J. H. Lipton, D. H. Vesole, et al. 2007. Posaconazole or fluconazole prophylaxis in severe graft versus host disease. N. Engl. J. Med. 35:335-347. [DOI] [PubMed] [Google Scholar]
- 96.Van de Velde, V. J., A. P. Van Peer, J. J. Heykants, et al. 1996. Effect of food on the pharmacokinetics of a new hydroxypropyl-beta-cyclodextrin formulation of itraconazole. Pharmacotherapy 16:424-428. [PubMed] [Google Scholar]
- 97.Van Peer, A., R. Woestenborghs, J. Heykants, et al. 1989. The effects of food and dose on the oral systemic availability of itraconazole in healthy subjects. Eur. J. Clin. Pharmacol. 36:423-426. [DOI] [PubMed] [Google Scholar]
- 98.Vermes, A., I. H. van der Sijs, and H. J. Guchelarr. 2000. Flucytosine: correlation between toxicity and pharmacokinetic parameters. Chemotherapy 46:86-94. [DOI] [PubMed] [Google Scholar]
- 99.Walsh, T. J., I. Raad, T. F. Patterson, et al. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]
- 100.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Arrieta, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single-or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiler, S., H. Zoller, I. Graziadei, W. Vogel, R. Bellmann-Weiler, M. Joannidis, et al. 2007. Altered pharmacokinetics of voriconazole in a patient with liver cirrhosis. Antimicrob. Agents Chemother. 51:3459-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yagasaki, K., S. Gando, N. Matsuda, T. Kameue, T. Ishitani, T. Hirano, and K. Iseki. 2003. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med. 29:1844-1848. [DOI] [PubMed] [Google Scholar]