Abstract
Burn tissue sites are a potential source of bacteremia during debridement surgery. Burn injury is likely to affect the distribution of antibiotics to tissues, but direct evidence of this is lacking. The aim of this study was to directly evaluate the influence of burn trauma on the distribution of cephalothin to peripheral tissues. We used subcutaneous microdialysis techniques to monitor interstitial fluid concentrations of cephalothin in the burnt and nonburnt tissues of adult patients with severe burns following parenteral administration of 1 g cephalothin for surgical prophylaxis. Analogous simultaneous studies conducted with healthy adult volunteers provided reference tissue concentration data. Equivalent tissue exposures were seen for burn and nonburn sites, giving overall median interstitial cephalothin concentrations (from 0 to 240 min) of 2.84 mg/liter and 3.06 mg/liter, respectively. A lower overall median interstitial cephalothin concentration of 0.54 mg/liter was observed for healthy individuals, and the patient nonburnt tissue and volunteer control tissue cephalothin concentrations exhibited significantly different data distributions (P < 0.001; Kolmogorov-Smirnov nonparametric test). The duration of tissue residence for cephalothin was longer for burn patients than for healthy volunteers. The results demonstrate the potential fallibility of using healthy population models to extrapolate tissue pharmacodynamic predictions from plasma data for burn patients.
Severe burns and their treatment result in local (17) and systemic (9, 22) pathophysiological responses that greatly influence the pharmacokinetics of drugs that are used intraoperatively (4, 7, 8, 12, 21). The predominant focus of previous investigations has been plasma antibiotic concentrations for the prevention of bacteremia. In burn patients, one of the sources of bacteremia is from the burn sites, and therefore consideration of antibiotic concentrations in the tissues becomes relevant. Antibiotic concentrations in blood plasma and interstitial fluid do not necessarily run parallel (10, 18), and blood-plasma-to-interstitial-fluid exchange is often tissue and drug specific (10, 13). Routine measurement of tissue antibiotic concentrations may not be practical in the clinical setting; however, the assessment of a relationship between plasma and interstitial levels of antibiotic may allow us to develop predictive models for tissue antibiotic concentrations from plasma measurements.
We used subcutaneous microdialysis techniques to monitor interstitial fluid concentrations of cephalothin in the burnt and nonburnt tissues of adult patients with severe burns following parenteral administration of 1 g cephalothin. Analogous studies conducted in healthy adult volunteers provided reference tissue concentration data. The study aim was to directly evaluate the influence of burn trauma on the distribution of cephalothin to peripheral tissues.
MATERIALS AND METHODS
Study design.
This open-label, nonrandomized study of cephalothin distribution into subcutaneous tissue was conducted with the burn and nonburn sites of six burn patients and the healthy forearms of five volunteers. Cephalothin was administered centrally by infusion and monitored peripherally by microdialysis, with correction for probe recovery by the method of retrodialysis by calibrator (1). Site and patient matched paired comparisons of burnt and nonburnt tissue cephalothin microdialysate levels were conducted. A nonpaired comparison of microdialysate levels from nonburnt tissue sites in burn patients and healthy volunteers was also undertaken.
Clinical context of the study.
Current local practice is for 1 g cephalothin to be administered <1 hour before debridement to provide broad-spectrum prophylactic cover, predominantly against methicillin- and penicillin-sensitive Staphylococcus aureus strains (19), for which the MIC of cephalothin has been reported to be 0.2 mg/liter (23). Interstitial concentrations of the MIC (0.2 mg/liter) and 5× MIC (1 mg/liter) were used merely for illustrative purposes in the current study.
Research context of the study.
The microdialysis data for this study were obtained in conjunction with our previous study reporting plasma levels (4). The same original cohort of nine burn patients was enrolled in both investigations, but three patients were withdrawn from the microdialysis study as a consequence of the considerable practical difficulties of conducting microdialysis in two body sites throughout eschar debridement and grafting procedures.
Ethical review.
The protocol received approval from the Hospital and University of Queensland Human Research Ethics Committees. Written informed consent was obtained from the legal guardians of enrolled patients and from the healthy volunteers.
Patient and volunteer enrollment.
Six adult patients with a mean (± standard deviation [SD]) age of 32 ± 11 years and total body surface area burns of 42% ± 9% were enrolled in the study. The patients were admitted to the Royal Brisbane & Women's Hospital intensive care unit between February 2005 and January 2006 and received eschar debridement and grafting surgery within the first few days postinjury, during which the studies were conducted. Exclusion criteria included an age of <18 years, existing bacterial infection, and known infection with hepatitis A, B, or C virus or human immunodeficiency virus. Patients were resuscitated during the burn shock phase, using the Parkland formula adjusted to patients' requirements (6).
Five volunteers with a mean (± SD) age of 35 ± 10 years were recruited exclusively from within the research group associated with the study. Exclusion criteria included an age of <18 years, poor health as assessed by a medical practitioner, contraindicated medications, and a known allergy to penicillin.
Burn patient and healthy volunteer study protocols.
Patient studies were conducted during debridement and grafting procedures within 4 days of trauma (mean posttrauma delay before grafting, 2.3 ± 1.1 days; mean surgery duration, 5.4 ± 1.3 h). First-dose cephalothin was investigated in all subjects. Patients received 1 g cephalothin in 10 ml 0.9% saline over 5 min through a dedicated lumen of a central venous catheter; volunteers received the same dose regimen intravenously into a forearm vein. Burn patient microdialysis sites were selected for anticipated ease of access during debridement surgery in body areas that were not expected to be required as skin graft donor sites or scheduled for eschar debridement at this procedure. Full-thickness burn sites and adjacent nonburnt skin areas in the neck/shoulder and groin/thigh areas were used. After insertion, probes were held in place with a surgical stitch and then, in the operating theater, were covered with protective sterile gauze and stapled to avoid dislodgment during the debridement procedure.
All volunteers received 1 g cephalothin in 10 ml 0.9% saline over 5 min through a peripheral venous catheter inserted into the basilic vein at the elbow. The microdialysis site for volunteers was the radial forearm on the side contralateral to the one used for the administration of cephalothin. Patient and volunteer microdialysis sites were anesthetized with 1% lignocaine (Xylocaine; AstraZenica, United Kingdom) before probe insertion. The probe was held in place with Tegaderm (3M Health Care, MN). CMA 60 microdialysis probes (CMA, Stockholm, Sweden) were perfused with aseptically prepared 0.9% saline containing 2 mg/liter cefazolin at a flow rate of 1.6 μl per minute from a 1-ml syringe, using a Graseby MS16 24-h syringe driver (Smiths Group Plc, London, United Kingdom). Microdialysis probes were perfused for up to 30 min prior to insertion to remove the preservative buffer. Probe perfusate was collected into sterile CMA collection vials, which were changed at 20-min intervals and stored on ice. Probe perfusate eluted prior to cephalothin administration was discarded. Experiments began upon commencement of cephalothin infusion, whose timing was dictated by the anticipated surgery commencement time, in accordance with local therapeutic guidelines for surgical site infection prophylaxis (19). Prior to storage at −20°C, microdialysis perfusate was carefully transferred to preweighed reduced-volume 300-μl polypropylene autosampler vials (Phenomenex, CA), using a refrigerated centrifuge. This enabled probe outflow volume measurement, ensured optimal sample storage conditions, and greatly facilitated subsequent sample handling.
Antibiotic analysis.
Microdialysate analysis by liquid chromatography-tandem mass spectrometry used an API 2000 mass spectrometer (Applied Biosystems) in positive electrospray mode (cephalothin Q1/Q3 = 397/152.2 m/z, cefazolin Q1/Q3 = 455.2/156 m/z, and benzylpenicillin Q1/Q3 = 335.1/160 m/z). Chromatographic separation on a 50- by 2-mm Gemini C18 column (Phenomenex) was achieved at a flow rate of 300 μl/min with a binary gradient (5 to 12 min postinjection) of 0% to 76% acetonitrile in 0.1% formic acid-water, using a Shimadzu high-performance liquid chromatography system, and gave retention times of 13.2, 12.0, and 12.6 min for cephalothin, cefazolin, and benzylpenicillin (internal standard), respectively. Calibration curves (0.1 to 16 μg/ml) were linear (r2 > 0.99). For sample preparation, 5 μl microdialysate and 70 μl benzylpenicillin (200 ng/ml in 0.9% saline) were mixed in polypropylene vials, and 50 μl was used for injection.
Microdialysis recovery correction.
The method of continuous retrodialysis by calibrator (1) was used to calculate microdialysis recovery for each data point, using measurements of cefazolin (calibrator) loss into tissue across the semipermeable membrane according to the following equation:
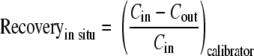 |
where Cin and Cout were the concentrations of cefazolin (calibrator) in the microdialysis perfusion fluid inflow and the microdialysis perfusate outflow, respectively. The mean recovery for each microdialysis probe was subsequently used to calculate cephalothin interstitial fluid concentrations.
Microdialysis data pairing.
A small number of burn patient microdialysis samples were lost as a result of temporary impairment of microdialysis probe perfusion. To ensure true pairing of the burn and nonburn microdialysis data, values corresponding to a lost sample pair were omitted.
Data analysis and presentation.
Mean (± SD) interstitial fluid concentrations of cephalothin and non-protein-bound (unbound) plasma cephalothin concentrations are presented as log-linear plots. Target microdialysis sample collection times were plotted. Minor variations in microdialysis sample times were unavoidable; 20-min-interval target times fell within the 95% confidence interval (95% CI) of each mean sample time. Linear interpolation of unbound plasma cephalothin concentrations permitted their temporal matching with the actual microdialysis sampling intervals. Unbound cephalothin data were calculated from patient and volunteer total plasma cephalothin values collected as described previously (4). Prestudy plasma samples were used for measurements of percent plasma protein binding of cephalothin. Briefly, 500 μl of 100-μg/ml cephalothin in plasma was incubated (37°C, 30 min) and ultracentrifuged (12,000 × g, 20 min) through 30-kDa-nominal-cutoff membrane devices (Amicon YM30; Millipore Corporation, Billerica, MA) to give a filtrate yield of approximately 25% of the original volume, which was analyzed by high-performance liquid chromatography (4). The times above the MIC of cephalothin against S. aureus (T > MICS. aureus) and five times the S. aureus MIC (T > 5× MICS. aureus) were obtained for a published MIC of 0.2 mg/liter (23). Values for T > MICS. aureus and T > 5× MICS. aureus were derived from terminal-phase log-linear interpolation of weighted (1/y) biexponential fits (C = Ae−αt + Be−βt) to each interstitial concentration-time data set. T > MICS. aureus and T > 5× MICS. aureus data are presented as column dot plots overlaid with mean and 95% CI marks. Computation made use of Microsoft Excel 2003 (Microsoft Corporation) for data analysis, Graphpad Prism for Windows, version 4.03 (Graphpad Software Inc.), for regression analysis, SPSS for Windows, version 15.0.1 (SPSS Inc.), for statistical analysis, and the R computing language to compile all plots (R Development Core Team [http://www.R-project.org]). Statistical testing of T < MICS. aureus values was done by Student's t test, applying pairing only to burn-nonburn paired comparisons. The highly robust Kolmogorov-Smirnov nonparametric distribution-independent test (KS test) was used for direct comparisons of site-itemized interstitial cephalothin data, unbound cephalothin data, and microdialysis probe recovery data.
RESULTS
Patient and volunteer demographics.
Table 1 shows the patient and volunteer demographic data, plasma protein binding of cephalothin, and patient surgery details. The two study cohorts were well matched in terms of body size and age.
TABLE 1.
Subject demographics and patient surgery details
| Characteristic | Mean ± SD for group
|
|
|---|---|---|
| Patients | Volunteers | |
| Age (yr) | 32 ± 11 | 35 ± 10 |
| Height (m) | 1.76 ± 0.08 | 1.73 ± 0.12 |
| Weight (kg) | 82.5 ± 14 | 68 ± 13 |
| TBSAa (%) | 41.6 ± 8.5 | |
| Posttrauma delay (days) | 2.3 ± 1.1 | |
| Surgery duration (h) | 5.4 ± 1.3 | |
| Vol (liters) of fluid administeredb | ||
| Intravenous crystalloid | 3.55 ± 2.37 | |
| Albumin | 0.67 ± 0.23 | |
| Blood products | 2.59 ± 1.97 | |
| Plasma bindingc (%) | 58.0 ± 9.0 | 70.8 ± 2.4 |
Total burn surface area (sum of second and third degree burn areas).
Itemized fluid volumes administered over 8 hours.
Plasma protein binding of cephalothin.
Paired comparison of interstitial cephalothin concentrations in burnt and nonburnt tissues.
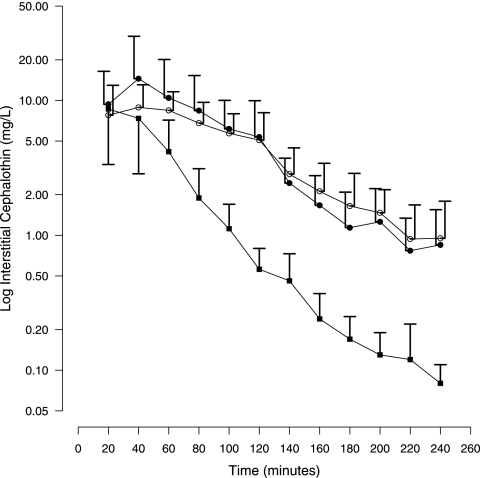
Figure 1 shows the patient matched paired comparison of mean interstitial cephalothin concentrations in burnt and nonburnt subcutaneous tissues in burn patients during debridement surgery. Equivalent tissue exposures were seen for burn and nonburn sites, giving overall median interstitial cephalothin concentrations (from 0 to 240 min) of 2.84 mg/liter and 3.06 mg/liter, respectively, with only 23% of the burn site data being <1 mg/liter (5× MICS. aureus). Burn site interstitial cephalothin concentrations of <0.2 mg/liter (MICS. aureus) were not observed in any patient within 240 min. No significant difference between the burn site and nonburn site interstitial cephalothin concentration data distributions was observed (P = 0.133; KS test).
FIG. 1.
Semilogarithmic plot of mean plus SD interstitial cephalothin concentrations for healthy volunteers (filled squares), burn patient nonburn sites (open circles), and burn patient burn sites (filled circles).
Comparison of interstitial cephalothin concentrations in nonburnt tissues of patients and healthy volunteers.
Figure 1 allows comparison of mean interstitial cephalothin concentrations in nonburnt tissues of burn patients and forearm sites of healthy volunteers. Nonburnt tissue exposure to cephalothin was higher for burn patients than for healthy individuals, giving overall median interstitial cephalothin concentrations (from 0 to 240 min) of 3.06 mg/liter and 0.54 mg/liter, respectively. The patient nonburnt tissue and volunteer control tissue cephalothin concentrations exhibited significantly different data distributions (P < 0.001; KS test). Higher peak concentrations were seen for nonburn sites than for controls, and the levels were maintained for longer times, such that only 7% of the nonburn site patient data were lower than 0.2 mg/liter (MICS. aureus), compared with 28% of the healthy control data. Similarly, 20% of the nonburn site patient data were lower than 1 mg/liter (5× MICS. aureus), compared with 64% of the healthy control data.
Comparison of interstitial cephalothin T > MICS. aureus values for burnt and nonburnt tissues of patients and control tissues of healthy volunteers.
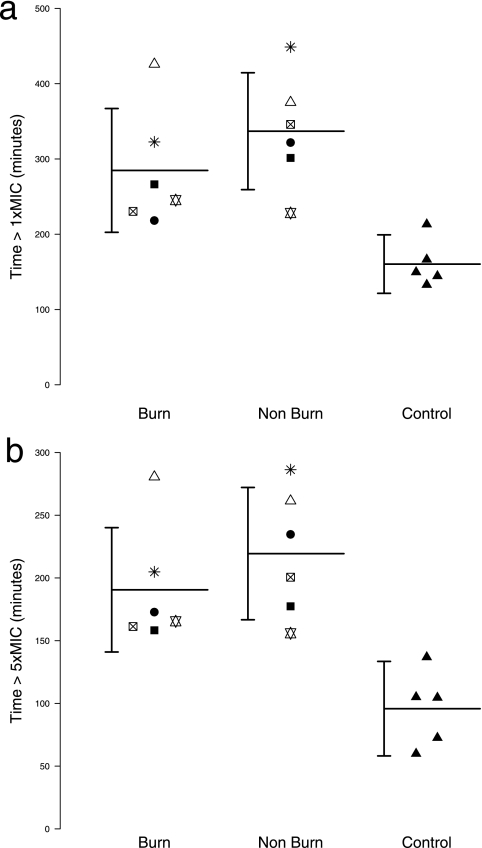
Figure 2a shows the T > MICS. aureus data for interstitial cephalothin in each study subject grouped by tissue site and overlaid by grouped means and 95% CI. Nonburnt tissue sites in burn patients gave significantly higher T > MICS. aureus values than did forearm sites in healthy volunteers (control), with times (mean ± 95% CI) of 336 ± 74 min and 160 ± 31 min, respectively (P = 0.001; nonpaired t test). Patient matched paired comparison of interstitial cephalothin T > MICS. aureus data revealed no mean difference between burn sites and nonburn sites (P = 0.148; paired t test), with burn sites giving a T > MICS. aureus (mean ± 95% CI) of 285 ± 78 min.
FIG. 2.
Column dot plots of T > MICS. aureus (a) and T > 5× MICS. aureus (b) data grouped by tissue site and overlaid with mean and 95% CI marks. T > MICS. aureus and T > 5× MICS. aureus values were derived from terminal-phase log-linear interpolation of weighted (1/y) biexponential fits (C = Ae−αt + Be−βt) to each interstitial concentration-time data set. Control and nonburn means differed significantly for both T > MICS. aureus (a) and T > 5× MICS. aureus (b) data (P = 0.001; nonpaired t test).
Comparison of interstitial cephalothin T > 5× MICS. aureus values for burnt and nonburnt tissues of patients and control tissues of healthy volunteers.
Figure 2b shows the T > 5× MICS. aureus data for interstitial cephalothin in each study subject grouped by tissue site and overlaid by grouped mean and 95% CI. Nonburnt tissue sites in burn patients gave significantly higher T > 5× MICS. aureus values than did forearm sites in healthy volunteers (control), with times (mean ± 95% CI) of 219 ± 53 min and 96 ± 38 min, respectively (P = 0.001; nonpaired t test). Patient matched paired comparison of interstitial cephalothin T > 5× MICS. aureus data revealed no mean difference between burn sites and nonburn sites (P = 0.135; paired t test), with burn sites giving a T > 5× MICS. aureus (mean ± 95% CI) of 191 ± 50 min.
Comparison of maximal unbound cephalothin concentrations in the plasma and interstitial compartments for patients and healthy volunteers.
Maximal plasma cephalothin concentrations are observed in burn patients upon completion of infusion (4). The mean ± SD postinfusion (5 min) unbound plasma cephalothin concentrations for healthy volunteers and burn patients were 17.6 ± 5.4 mg/liter and 38.7 ± 19.7 mg/liter, respectively. For healthy volunteers, maximal mean interstitial concentrations of unbound cephalothin were captured in the first microdialysis collection (0 to 20 min) and gave a mean ± SD of 8.6 ± 5.3 mg/liter. The second microdialysis collection (20 to 40 min) captured maximal mean interstitial concentrations of unbound cephalothin for burn patients and gave values of 8.9 ± 4.2 mg/liter and 14.5 ± 15.4 mg/liter for nonburn and burn sites, respectively.
Comparison of interstitial cephalothin and unbound plasma cephalothin in burnt and nonburnt tissues of patients and control tissues of healthy volunteers.
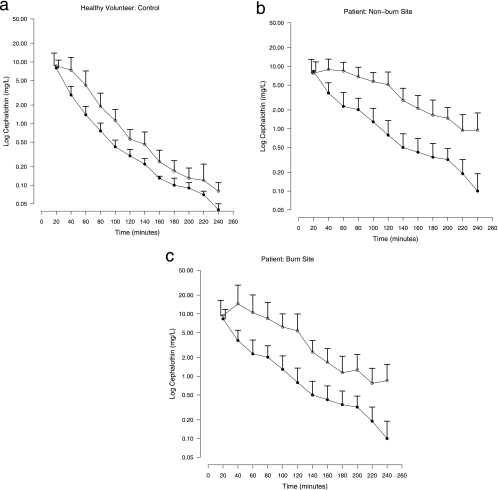
Interstitial cephalothin and unbound plasma cephalothin concentrations for patients and volunteers are presented in Fig. 3. Volunteer control interstitial cephalothin and unbound cephalothin concentrations ran parallel (Fig. 3a) and gave equivalent data distributions (P = 0.4; KS test). Burn patient nonburn site (Fig. 3b) and burn site (Fig. 3c) interstitial cephalothin concentration profiles lagged behind unbound plasma cephalothin concentrations, with higher concentrations being maintained within the interstitium. Patient nonburn and burn interstitial cephalothin concentration data distributions were both distinct from the corresponding unbound plasma cephalothin data distribution (P < 0.001; KS test).
FIG. 3.
Mean plus SD interstitial cephalothin (open circles) and unbound plasma cephalothin (closed circles) concentrations for healthy volunteers (a), patient nonburn sites (b), and patient burn sites (c). Error bars for interstitial and unbound plasma cephalothin values are displaced to the left and right, respectively, for 20-min data only. Temporal matching of plasma values to microdialysis sample times was achieved by linear interpolation. The unbound plasma cephalothin value was calculated with prestudy plasma protein binding data for each subject.
Ratio of interstitial cephalothin to unbound plasma cephalothin in burnt and nonburnt tissues of patients and control tissues of healthy volunteers.
Patient matched paired burn and nonburn site investigations gave overall (0 to 240 min) mean ± SD interstitial/unbound plasma cephalothin ratios of 8.56 ± 12.0 and 7.77 ± 10.3, respectively. No significant difference in the data distributions of the interstitial/unbound plasma cephalothin ratio data was seen between the burn and nonburn sites (P = 0.64; KS test). The ratios of interstitial cephalothin to unbound plasma cephalothin concentrations were consistently lower for the volunteers than for the nonburn sites of burn patients (P < 0.001; KS test), giving an overall (0 to 240 min) mean ± SD ratio of 2.0 ± 1.2.
Microdialysis probe recovery details.
Probe recovery data for patients and volunteers were constant with time. The mean microdialysis recovery data for all patient (n = 122) and volunteer (n = 54) microdialysis measurements were 44% ± 20% and 68% ± 16%, respectively. Patient and volunteer microdialysis probe recovery data distributions differed significantly (P < 0.001; KS test). Significantly different microdialysis probe recovery data distributions were also observed within the burn patient cohort, with mean burn site (n = 61) and nonburn site (n = 61) recoveries being 38% ± 18% and 50% ± 19%, respectively (P < 0.01; KS test). The relative order of microdialysis probe recoveries was as follows: healthy volunteer > patient nonburn site > patient burn site.
DISCUSSION
The principal findings of this study were that a single bolus dose of 1 g of cephalothin gave equivalent cephalothin kinetics in the subcutaneous tissues of burn sites and nonburn sites of burn patients and that interstitial cephalothin concentrations were maintained longer in burn patients than in healthy volunteers. The MIC of cephalothin against methicillin- and penicillin-sensitive Staphylococcus aureus strains was exceeded for over 4 h in burn patient tissues.
This study is the first to apply microdialysis to a comparison of interstitial antibiotic concentrations in a surgical patient group and a nonsurgical volunteer group. It is also the first reported patient matched paired comparison of burn site and nonburn site interstitial antibiotic concentrations in an acute-phase burn patient population. The reported data are of direct clinical relevance, having been derived using established microdialysis techniques coupled with robust and effective calibration methods (retrodialysis by calibrator) (1) and validated analysis protocols. Stringent selection criteria were applied to the burn cohort; we studied first-dose cephalothin kinetics during debridement surgery within 4 days of trauma in patients with >30% total body surface area burns and no signs of infection.
We previously published guidance on prophylaxis of transient bacteremia, indicating the need for intraoperative redosing of cephalothin during prolonged surgery for maintenance of effective plasma concentrations (4). Burnt tissue is one source of bacteremia during burn debridement, and therefore consideration of antibiotic concentrations in the tissues is also relevant. The results of the current study suggest that the pathophysiological determinants of altered antibiotic distribution in burn patients actually create a favorable environment for antimicrobial action in tissue sites. Evidence for this can be seen from the plots of interstitial cephalothin T > MICS. aureus data displayed in Fig. 2 and from the comparisons of interstitial cephalothin time courses for nonburn sites in patients and volunteers given in Fig. 1. The underlying causes of prolonged tissue residence times of cephalothin in burn patient tissues appear to be complex in nature, affecting both burn and nonburn sites to similar extents (Fig. 1 and 2).
Accurate measurement of interstitial drug concentrations by microdialysis is reliant on appropriate calibration methodology (2). The current investigation used the method of continuous retrodialysis by calibrator (1) and met all relevant technical prerequisites (1, 2). It is of considerable interest that the microdialysis probe recovery data were affected by the tissue site. The relative order of microdialysis probe recoveries was as follows: healthy volunteer control site > patient nonburn site > patient burn site. Low microdialysis probe recoveries result from a small loss of calibrator (cefazolin) from the microdialysis probe and may be a consequence of impaired tissue clearance of cefazolin from burnt and, to a lesser extent, nonburnt tissues.
The rates of disappearance for unbound cephalothin concentrations in plasma and interstitial fluid appeared to be parallel in the volunteer group, consistent with an elimination clearance with a slight delay in the interstitial profile, reflecting a delay in the transfer between the plasma and interstitial compartments. In contrast, the unbound profiles for the interstitial fluid were significantly higher than the unbound plasma concentrations, but the disappearance rates were similar after 40 min for the burn sites and after 100 min for the nonburn sites (Fig. 3). The longer delay between unbound cephalothin concentration equivalence in the plasma and interstitium observed for burn patients than for healthy volunteers is consistent with reports of impaired full-thickness burn tissue vascular perfusion (3). The similar increases in the area under the curve for unbound interstitial levels in patient nonburnt and burnt tissue sites probably arose in part from bound cephalothin (plasma protein binding in burn patients, 58% ± 9%) being carried into the tissues with the albumin that undergoes extravasation, as reported for patients with severe edema (11). All recruited patients exhibited systemic inflammatory response syndrome, which results in microvascular leakage of plasma proteins and fluid into the interstitium. Furthermore, systemic endothelial dysfunction is exacerbated during burn debridement (20) as a result of localized release of inflammatory factors from manipulated burn tissue (14). Increased areas under the curve for unbound interstitial levels in the nonburnt and burnt tissue sites are also in keeping with prior published observations of increased antibiotic distribution volumes and third-space sequestration seen in patients with severe edema (15, 16), an increased extravascular space, and impaired or delayed lymphatic drainage (5).
This study has shown equivalent distributions of cephalothin to burnt and nonburnt tissue sites and illustrated the maintenance of elevated tissue levels for longer in burn patients than in healthy volunteers. The results demonstrate the potential fallibility of extrapolating tissue pharmacodynamic predictions from plasma data for burn patients in the same way as those extrapolated between compartments in the healthy population.
Acknowledgments
This work was funded by National Health and Medical Research Council of Australia grant 351519.
We gratefully acknowledge Michael Steyn for practical assistance with microdialysis during debridement procedures and Melissa Rutt for her technical laboratory assistance.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Bouw, M. R., and M. Hammarlund-Udenaes. 1998. Methodological aspects of the use of a calibrator in in vivo microdialysis—further development of the retrodialysis method. Pharm. Res. 15:1673-1679. [DOI] [PubMed] [Google Scholar]
- 2.Chaurasia, C. S., M. Muller, E. D. Bashaw, E. Benfeldt, J. Bolinder, R. Bullock, P. M. Bungay, E. C. M. DeLange, H. Derendorf, W. F. Elmquist, M. Hammarlund-Udenaes, C. Joukhadar, D. L. Kellogg, C. E. Lunte, C. H. Nordstrom, H. Rollema, R. J. Sawchuk, B. W. Y. Cheung, V. P. Shah, L. Stahle, U. Ungerstedt, D. F. Welty, and H. Yeo. 2007. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm. Res. 24:1014-1025. [DOI] [PubMed] [Google Scholar]
- 3.Cross, K. M., L. Leonardi, J. R. Payette, M. Gomez, M. A. Levasseur, B. J. Schattka, M. G. Sowa, and J. S. Fish. 2007. Clinical utilization of near-infrared spectroscopy devices for burn depth assessment. Wound Repair Regen. 15:332-340. [DOI] [PubMed] [Google Scholar]
- 4.Dalley, A. J., J. Lipman, B. Venkatesh, M. Rudd, M. S. Roberts, and S. E. Cross. 2007. Inadequate antimicrobial prophylaxis during surgery: a study of beta-lactam levels during burn debridement. J. Antimicrob. Chemother. 60:166-169. [DOI] [PubMed] [Google Scholar]
- 5.Demling, R. H. 2005. The burn edema process: current concepts. J. Burn Care Rehabil. 26:207-227. [PubMed] [Google Scholar]
- 6.Dulhunty, J. M., R. J. Boots, M. J. Rudd, M. J. Muller, and J. Lipman. 2008. Increased fluid resuscitation can lead to adverse outcomes in major-burn injured patients, but low mortality is achievable. Burns 34:1090-1097. [DOI] [PubMed] [Google Scholar]
- 7.Han, T. H., J. S. Harmatz, D. J. Greenblatt, and J. A. J. Martyn. 2007. Fentanyl clearance and volume of distribution are increased in patients with major burns. J. Clin. Pharmacol. 47:674-680. [DOI] [PubMed] [Google Scholar]
- 8.Han, T. H., J. H. Lee, I. S. Kwak, H. Y. Kil, K. W. Han, and K. M. Kim. 2005. The relationship between bispectral index and targeted propofol concentration is biphasic in patients with major burns. Acta Anaesthesiol. Scand. 49:85-91. [DOI] [PubMed] [Google Scholar]
- 9.Jaehde, U., and F. Sörgel. 1995. Clinical pharmacokinetics in patients with burns. Clin. Pharmacokinet. 29:15-28. [DOI] [PubMed] [Google Scholar]
- 10.Lin, J. H. 2006. Tissue distribution and pharmacodynamics: a complicated relationship. Curr. Drug Metab. 7:39-65. [DOI] [PubMed] [Google Scholar]
- 11.Lund, T., H. Onarheim, and R. K. Reed. 1992. Pathogenesis of edema formation in burn injuries. World J. Surg. 16:2-9. [DOI] [PubMed] [Google Scholar]
- 12.Macfie, A. G., A. D. Magides, and C. S. Reilly. 1992. Disposition of alfentanil in burns patients. Br. J. Anaesth. 69:447-450. [DOI] [PubMed] [Google Scholar]
- 13.Muller, M., A. D. Pena, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48:1441-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papini, R. P., A. P. R. Wilson, J. A. Steer, G. Hill, D. A. McGrouther, and N. Parkhouse. 1997. Plasma concentrations of tumour necrosis factor-alpha and interleukin-6 during burn wound surgery or dressing. Br. J. Plast. Surg. 50:354-361. [DOI] [PubMed] [Google Scholar]
- 15.Pea, F., P. Viale, and M. Furlanut. 2005. Antimicrobial therapy in critically ill patients—a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 44:1009-1034. [DOI] [PubMed] [Google Scholar]
- 16.Pinder, M., R. Bellomo, and J. Lipman. 2002. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth. Intensive Care 30:134-144. [DOI] [PubMed] [Google Scholar]
- 17.Samuelsson, A., I. Steinvall, and F. Sjöberg. 2006. Microdialysis shows metabolic effects in skin during fluid resuscitation in burn-injured patients. Crit. Care 10:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente, M. V., T. Olay, M. C. R. Quecedo, and A. Rodriguez. 1979. Diffusion of beta-lactam antibiotics and fosfomycin to interstitial-tissue fluid in rabbits. Chemotherapy 25:329-335. [DOI] [PubMed] [Google Scholar]
- 19.Victorian Drug Usage Advisory Committee. 2006. Therapeutic guidelines: antibiotic, 13th ed. Victorian Drug Usage Advisory Committee, Melbourne, Australia.
- 20.Vlachou, E., P. Gosling, and N. S. Moiemen. 2008. Microalbuminuria: a marker of systemic endothelial dysfunction during burn excision. Burns 34:241-246. [DOI] [PubMed] [Google Scholar]
- 21.Walstad, R. A., L. Aanderud, and E. Thurmann-Nielsen. 1988. Pharmacokinetics and tissue concentrations of ceftazidime in burn patients. Eur. J. Clin. Pharmacol. 35:543-549. [DOI] [PubMed] [Google Scholar]
- 22.Weinbren, M. J. 1999. Pharmacokinetics of antibiotics in burn patients. J. Antimicrob. Chemother. 44:319-327. [DOI] [PubMed] [Google Scholar]
- 23.Williams, J. D., and F. Moosdeen. 1987. In-vitro antibacterial effects of cephalosporins. Drugs 34:44-63. [DOI] [PubMed] [Google Scholar]