Abstract
We identified a 6-aminoquinolone compound, WC5, that inhibits human cytomegalovirus (HCMV) replication with a selectivity index of ∼500. WC5 also showed activity against drug-resistant HCMV strains. In contrast, it did not significantly affect the replication of human herpesvirus 6 and 8 and was ∼10-fold less active against murine cytomegalovirus. Thus, WC5 may represent a lead for the development of new, potent, and selective anti-HCMV compounds.
Human cytomegalovirus (HCMV) infects between 60 and 90% of the world's population, depending on socioeconomic class and geographic location (10). It is a serious, life-threatening, opportunistic pathogen in immunocompromised individuals, such as AIDS patients and organ transplant recipients, who are at great risk of developing severe diseases such as pneumonia, gastrointestinal disease, and retinitis (6); in addition, intrauterine HCMV infection is the leading cause of congenital malformation in newborn children (10). In spite of the biomedical importance of HCMV infection for at-risk populations, we have yet to develop adequate antiviral strategies. Indeed, although there are a few anti-HCMV agents, including ganciclovir (GCV), foscarnet (phosphonoformic acid [PFA]), and cidofovir (CDV), they are all limited by toxicity, viral resistance, and pharmacokinetic drawbacks (5). Thus, there is still a considerable need for new anti-HCMV drugs.
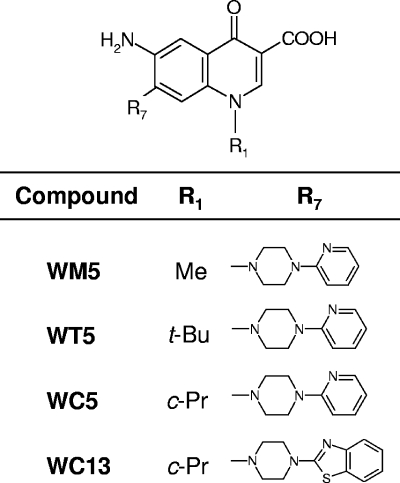
Quinolones, whose main structural feature is a 1,4-dihydro-4-oxo-pyridinyl moiety bearing an essential carboxyl group at the C-3 position, were first reported as an important class of broad-spectrum antibacterials able to inhibit prokaryotic type II topoisomerases (1). Later, several quinolone derivatives were shown to possess antiviral activity (12); in particular, properly functionalized 6-fluoroquinolones (2, 3, 7) as well as 6-aminoquinolones (6-AQs) (4, 18), which are characterized by an amino group at the C-6 position of the bicyclic quinolone ring system, were shown to inhibit human immunodeficiency virus (HIV) replication. Among the 6-AQs, we previously identified WM5 (Fig. 1), which bears a methyl group at the N-1 position and a 4-(2-pyridyl)-1-piperazine moiety at the C-7 position, with potent anti-HIV activity in both acutely and chronically infected cells (4, 9, 18). Successively, other 6-AQs, of which one of the most potent was WC13 (Fig. 1), were shown to possess broad-spectrum antiviral properties, being able to inhibit HCMV in addition to HIV replication (14). We thus wished to investigate whether WM5 might also exhibit anti-HCMV activity.
FIG. 1.
Chemical structures of the 6-AQ derivatives considered in this study. Compounds were synthesized as previously described (4, 18). WM5, WT5, and WC5 correspond to compounds 12a, 7a, and 8a, respectively, reported in reference 4, while WC13 is compound 28d reported in reference 18. Me, methyl; t-Bu, tert-butyl; c-Pr, cyclopropyl.
The effect of WM5 on the replication of HCMV AD169 (purchased from the American Type Culture Collection [ATCC], Manassas, VA) in human foreskin fibroblast (HFF) cells was evaluated after a 10-day incubation by plaque reduction assays as described previously (8) and compared to that of two 6-AQ derivatives with similar structures, WT5 and WC5 (Fig. 1), which maintain the same 4-arylpiperazine substituent of WM5 at the C-7 position but bear a tert-butyl and a cyclopropyl, respectively, instead of a methyl group at the N-1 position (4). WM5 activity was also compared to that of WC13, one of the formerly reported 6-AQ derivatives with broad-spectrum antiviral properties (14), in which the C-7 pyridinylpiperazine substituent was modified by replacing the pyridine ring at the N-4 piperazine core with a benzothiazole group (18). In parallel, we tested the cytotoxicity of all compounds in HFF cells after 5 days by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) assays as described previously (8). GCV (purchased from Sigma) was included as a control in all experiments. Both WM5 and WC5 inhibited HCMV replication by 50% (50% effective concentration [EC50]) at submicromolar concentrations (EC50s were 0.7 μM and 0.9 μM, respectively), while WT5 did not exhibit significant activity (EC50 was 36.7 μM) (Table 1). The 50% cytotoxic concentration (CC50) of WM5 and WC5 in HFF cells was 54 μM and 431 μM, respectively, resulting in a selectivity index (SI = CC50/EC50) of 77 and 479, respectively (Table 1). As previously reported (14), WC13 showed activity against HCMV at a concentration that was ∼200-fold lower than the cytotoxic concentration (Table 1). As well as for the CC50 values determined by MTT assays, the 50% cytostatic concentration and the minimal cytotoxic concentration of WC5 (≥50 μM and ≥100 μM, respectively), determined as described in reference 14, compared favorably with those previously reported for WC13 (0.018 μM and ≥4 μM, respectively) (14). Thus, WC5 and WC13 appeared to be the most promising compounds among those tested, exhibiting an SI value similar to or higher than that of GCV. Remarkably, our data also demonstrate that as previously reported (13, 18), structural modifications at both the N-1 and C-7 position of the 6-AQ scaffold have a dramatic effect on the potency and selectivity of these agents, as they appear to modulate both their cytotoxicity and antiviral activity.
TABLE 1.
Antiviral activity against HCMV and cytotoxicity of 6-AQ compounds
| Compound | EC50 (μM)a | CC50 (μM)b | SIc |
|---|---|---|---|
| WM5 | 0.7 ± 0.2 | 54 ± 15 | 77 |
| WT5 | 36.7 ± 15.2 | ≥475 | ≥13 |
| WC5 | 0.9 ± 0.2 | 431 ± 61 | 479 |
| WC13 | 0.02 ± 0.05 | 4.5 ± 3.9 | 225 |
| GCV | 1.9 ± 0.2 | 550 ± 75 | 289 |
EC50 was determined by plaque reduction assays against HCMV AD169 in HFF cells. Reported values represent the means ± standard deviations (SD) of data derived from at least three independent experiments performed in duplicate.
CC50 was determined by MTT assays in HFF cells. Reported values represent the means ± SD of data derived from at least three independent experiments performed in quadruplicate.
SI was determined as the ratio between CC50 and EC50.
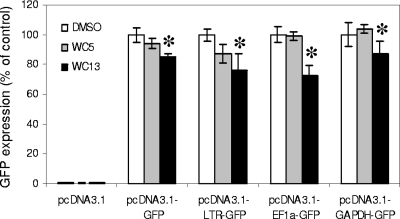
Previously, WC13 has been shown to inhibit gene expression mediated from different promoters, namely the HIV-1 long terminal repeat (LTR), HCMV immediate early (IE), and human elongation factor 1 alpha (EF-1α) gene promoters, in green fluorescent protein (GFP) transactivation experiments (14). To investigate whether the anti-HCMV activity of WC5 could also be ascribed to a transactivation-interfering process, we analyzed the effect of WC5 on GFP expression mediated from different viral and cellular promoters. 293T cells were transfected with pcDNA3.1-based plasmids containing the GFP reporter gene under the control of the HCMV IE (pcDNA3.1-GFP), HIV-1 LTR (pcDNA3.1-LTR-GFP), human EF-1α (pcDNA3.1-EF-1α-GFP), or human glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) gene (pcDNA3.1-GAPDH-GFP) promoter and treated for 48 h with either 0.2% dimethyl sulfoxide (DMSO) as a control or 50 μM WC5 or 0.5 μM WC13. Such compound concentrations were selected because they are not cytotoxic but are able to completely inhibit HCMV replication in HFF cells. GFP expression was analyzed by flow cytometry (FACSCalibur 3CA; Becton Dickinson). As previously reported (14), WC13 caused a decrease in GFP expression driven from all four promoters compared to the DMSO-treated control cells (Fig. 2). In contrast, WC5 did not inhibit GFP expression from any of the tested promoters, since GFP expression levels were comparable to those of control cells (Fig. 2). Similar results were obtained in cells cotransfected with an HIV-1 Tat-expressing plasmid (data not shown). Thus, WC5 seems to not possess broad-spectrum transactivation-interfering properties such as those of WC13, at least in uninfected cells.
FIG. 2.
Effect of WC5 and WC13 on transactivation of a reporter gene transcribed from viral and cellular promoters. 293T cells were transfected with plasmids bearing the GFP reporter gene under the control of HCMV IE (pcDNA3.1-GFP), HIV-1 LTR (pcDNA3.1-LTR-GFP), human EF-1α gene (pcDNA3.1-EF1α-GFP) or human GAPDH gene (pcDNA3.1-GAPDH-GFP) promoter, or pcDNA3.1 as a control. Cells were then incubated for 48 h with 50 μM WC5 (gray bars), 0.5 μM WC13 (black bars), or 0.2% DMSO as a control (white bars). Quantification of GFP expression was performed by flow cytometry. The data shown represent the means ± standard deviations (error bars) of three independent experiments. The asterisks denote a statistically significant difference (P < 0.05) between the values relative to the compound-treated samples and the values relative to the respective DMSO-treated sample.
To further evaluate the therapeutic potential and selectivity of WC5, we tested its effects on the replication of other herpesviruses, i.e., human herpesvirus 6 (HHV-6) and 8 (HHV-8) and murine cytomegalovirus (MCMV). A previous study already showed that WC5 is not significantly active against herpes simplex virus (4). The antiviral activity of WC5 against the A and B variants of HHV-6 (kindly provided by L. Naesens, Rega Institute for Medical Research, Leuven, Belgium) was determined in HSB-2 and MOLT-3 cells, respectively, by both microscopic estimation of the cytopathic effect (data not shown) and quantification of viral DNA replication at 12 days postinfection (Table 2) by quantitative real-time PCR (qPCR) as described previously (19). Overall, the EC50s obtained by the cytopathic effect and qPCR assay were very similar. The ability of WC5 to inhibit the lytic replication of HHV-8 upon induction with 12-O-tetradecanoylphorbol-13-acetate (Sigma) in latently infected BC-3 cells (from ATCC) was evaluated by quantification of viral DNA at 7 days postinfection by qPCR as described previously (20). Finally, WC5 activity against MCMV (strain Smith, kindly provided by D. Lembo, University of Turin, Italy) in NIH 3T3 cells was assayed by plaque reduction assays. The cytotoxicity of WC5 in all cell lines was assessed by MTT assays. The antiherpetic drugs PFA (from Sigma), GCV, and CDV (from Pfizer) were used as reference compounds. As shown in Table 2, the EC50s obtained for WC5 against both HHV-6 variants were in the same range (EC50s were 5.1 μM for HHV-6A and 3.3 μM for HHV-6B). The CC50s in HSB-2 and MOLT-3 cells were 32 μM and 24 μM, respectively, resulting in very low SI values. Thus, it is very likely that the effects of WC5 observed in antiviral assays are due to cytotoxicity rather than to specific antiviral activity, and hence, WC5 has little or no activity against HHV-6. Similar results were obtained with HHV-8 (Table 2). Moreover, WC5 was ∼10-fold less active against MCMV than against HCMV (an EC50 of 10.8 versus 0.9 μM). Thus, among the tested herpesviruses, the 6-AQ derivative WC5 exhibits the highest activity against HCMV.
TABLE 2.
Antiviral activity of WC5 against other herpesviruses
| Virus (strain) | Cell line | Controla
|
WC5
|
||||
|---|---|---|---|---|---|---|---|
| EC50 (μM)b | CC50 (μM)c | SId | EC50 (μM)b | CC50 (μM)c | SId | ||
| HHV-6A (GS) | HSB-2 | 6.6 ± 3.0 | 800 ± 150 | 121 | 5.1 ± 1.6 | 32 ± 6 | 6 |
| HHV-6B (Z29) | MOLT-3 | 4.3 ± 2.5 | 1,100 ± 150 | 256 | 3.3 ± 1.8 | 24 ± 8 | 7 |
| HHV-8 | BC-3 | 6.5 ± 1.2 | 256 ± 100 | 39 | 14.2 ± 7.3 | 47 ± 13 | 3 |
| MCMV (Smith) | NIH 3T3 | 0.4 ± 0.1 | 250 ± 10 | 625 | 10.8 ± 5.5 | 275 ± 25 | 25 |
For HHV-6A and HHV-6B, PFA was used; for HHV-8, GCV was used; and for MCMV, CDV was used.
EC50 was determined by quantification of viral DNA production by qPCR for HHV-6A, HHV-6B, and HHV-8 and by plaque reduction assays for MCMV. Reported values represent the means ± standard deviations (SD) of data derived from at least three independent experiments performed in duplicate.
CC50 was determined by MTT assays. Reported values represent the means ± SD of data derived from at least three independent experiments performed in quadruplicate.
SI was determined as the ratio between CC50 and EC50.
In the next series of experiments, plaque reduction assays were used to test the activity of WC5 both against a non-AD169 HCMV laboratory strain (Towne, purchased from ATCC) and against three clinical HCMV isolates (recovered from a pregnant woman, a patient with AIDS, and a lung transplant recipient). As well as with HCMV AD169, the EC50s obtained with WC5 compared favorably to those observed for GCV (EC50s were 2.1 μM for WC5 and 3.7 μM for GCV against the Towne strain; EC50s were 1.8 to 2.7 μM for WC5 and 1.7 to 4.5 μM for GCV against field isolates). In addition, we tested WC5 activity against a panel of viruses (all obtained from the NIH AIDS Research and Reference Reagent Program, Rockville, MD) with drug resistance mutations. WC5 retained its activity against HCMV strains resistant to GCV, CDV, PFA, and acyclovir (Table 3), suggesting that its mechanism of action most likely differs from that of those HCMV DNA polymerase inhibitors. Given the ability of quinolones to interact with nucleic acids isolated or complexed to proteins (1, 9, 11), a similar mechanism of action on nucleic acid-protein complexes may also be relevant for WC5 antiviral activity. Studies to elucidate the mechanism of action of WC5 will be necessary to test this hypothesis.
TABLE 3.
Antiviral activity of WC5 against drug-resistant HCMV strains
| HCMV strain | Site of mutation | Amino acid (aa) substitution or deletion | Resistance | Anti-HCMV agent | EC50 (μM)a
|
|
|---|---|---|---|---|---|---|
| Control | WC5 | |||||
| AD169 | None | None | None | GCV | 1.9 ± 0.2 | 0.9 ± 0.2 |
| None | None | None | PFA | 38.0 ± 3.5 | 0.9 ± 0.2 | |
| 759rD100b | UL97 kinase, DNA polymerase | 4-aa deletion (638-641), A987G | GCV | GCV | 11.5 ± 2.0 | 0.9 ± 0.1 |
| PFArD100c | DNA polymerase | Not determined | PFA, acyclovir | PFA | 300 ± 25 | 1.1 ± 0.1 |
| GDGrK17d | UL97 kinase | 4-aa deletion (638-641) | GCV | GCV | 15.3 ± 1.0 | 1.5 ± 0.5 |
| GDGrP53b | DNA polymerase | A987G | CDV, GCV | GCV | 11.5 ± 2.5 | 1.0 ± 0.1 |
In conclusion, the data presented here show that the 6-AQ derivative WC5 is a potent and specific inhibitor of HCMV, and thus, it may represent a promising lead for the development of a new class of effective anti-HCMV drugs.
Acknowledgments
We thank Lieve Naesens for kindly providing the HHV-6A and HHV-6B and David Lembo for supplying the MCMV.
This work was supported by PRIN 2005 (grant no. 2005060941), MURST (ex 60%), and Progetto di Ricerca di Ateneo 2007 (grant no. CPDA074945) to A.L., by Regione Veneto and Istituto Superiore di Sanità of Italy (grants 40G.44 and 30G.55) to G.P., and in part by PRIN 2006 (grant no. 2006030809) to O.T. and V.C.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Andriole, V. T. 1988. The quinolones. Academic Press, London, UK.
- 2.Baba, M., M. Okamoto, M. Kawamura, M. Makino, T. Higashida, T. Takashi, Y. Kimura, T. Ikeuchi, T. Tetsuka, and T. Okamoto. 1998. Inhibition of human immunodeficiency virus type 1 replication and cytokine production by fluoroquinoline derivatives. Mol. Pharmacol. 53:1097-1103. [PubMed] [Google Scholar]
- 3.Baba, M., M. Okamoto, M. Makino, Y. Kimura, T. Ikeuchi, T. Sakaguchi, and T. Okamoto. 1997. Potent and selective inhibition of human immunodeficiency virus type 1 transcription by piperazinyloxoquinoline derivatives. Antimicrob. Agents Chemother. 41:1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchetti, V., C. Parolin, S. Moro, T. Pecere, E. Filipponi, A. Calistri, O. Tabarrini, B. Gatto, M. Palumbo, A. Fravolini, and G. Palu. 2000. 6-Aminoquinolones as new potential anti-HIV agents. J. Med. Chem. 43:3799-3802. [DOI] [PubMed] [Google Scholar]
- 5.Coen, D. M., and P. A. Schaffer. 2003. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2:278-288. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths, P. D., and S. Walter. 2005. Cytomegalovirus. Curr. Opin. Infect. Dis. 18:241-245. [DOI] [PubMed] [Google Scholar]
- 7.Hagihara, M., H. Kashiwase, T. Katsube, T. Kimura, T. Komai, K. Momota, T. Ohmine, T. Nishigaki, S. Kimura, and K. Shimada. 1999. Synthesis and anti-HIV activity of arylpiperazinyl fluoroquinolones: a new class of anti-HIV agents. Bioorg. Med. Chem. Lett. 9:3063-3068. [DOI] [PubMed] [Google Scholar]
- 8.Loregian, A., and D. M. Coen. 2006. Selective anti-cytomegalovirus compounds discovered by screening for inhibitors of subunit interactions of the viral polymerase. Chem. Biol. 13:191-200. [DOI] [PubMed] [Google Scholar]
- 9.Parolin, C., B. Gatto, C. Del Vecchio, T. Pecere, E. Tramontano, V. Cecchetti, A. Fravolini, S. Masiero, M. Palumbo, and G. Palu. 2003. New anti-human immunodeficiency virus type 1 6-aminoquinolones: mechanism of action. Antimicrob. Agents Chemother. 47:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 11.Richter, S., C. Parolin, B. Gatto, C. Del Vecchio, E. Brocca-Cofano, A. Fravolini, G. Palú, and M. Palumbo. 2004. Inhibition of human immunodeficiency virus type 1 Tat-trans-activation-responsive region interaction by an antiviral quinolone derivative. Antimicrob. Agents Chemother. 48:1895-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter, S., C. Parolin, M. Palumbo, and G. Palu. 2004. Antiviral properties of quinolone-based drugs. Curr. Drug Targets Infect. Disord. 4:111-116. [DOI] [PubMed] [Google Scholar]
- 13.Richter, S. N., B. Gatto, O. Tabarrini, A. Fravolini, and M. Palumbo. 2005. Antiviral 6-amino-quinolones: molecular basis for potency and selectivity. Bioorg. Med. Chem. Lett. 15:4247-4251. [DOI] [PubMed] [Google Scholar]
- 14.Stevens, M., J. Balzarini, O. Tabarrini, G. Andrei, R. Snoeck, V. Cecchetti, A. Fravolini, E. De Clercq, and C. Pannecouque. 2005. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 56:847-855. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan, V., K. K. Biron, C. Talarico, S. C. Stanat, M. Davis, L. M. Pozzi, and D. M. Coen. 1993. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob. Agents Chemother. 37:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan, V., and D. M. Coen. 1991. Isolation of foscarnet-resistant human cytomegalovirus patterns of resistance and sensitivity to other antiviral drugs. J. Infect. Dis. 164:781-784. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 359:85. [DOI] [PubMed] [Google Scholar]
- 18.Tabarrini, O., M. Stevens, V. Cecchetti, S. Sabatini, M. Dell'Uomo, G. Manfroni, M. Palumbo, C. Pannecouque, E. De Clercq, and A. Fravolini. 2004. Structure modifications of 6-aminoquinolones with potent anti-HIV activity. J. Med. Chem. 47:5567-5578. [DOI] [PubMed] [Google Scholar]
- 19.Watzinger, F., M. Suda, S. Preuner, R. Baumgartinger, K. Ebner, L. Baskova, H. G. Niesters, A. Lawitschka, and T. Lion. 2004. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J. Clin. Microbiol. 42:5189-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White, I. E., and T. B. Campbell. 2000. Quantitation of cell-free and cell-associated Kaposi's sarcoma-associated herpesvirus DNA by real-time PCR. J. Clin. Microbiol. 38:1992-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]