Abstract
Three separate controlled, two-period studies with healthy volunteers assessed the pharmacokinetic interactions between tipranavir-ritonavir (TPV/r) in a 500/200-mg dose and 500 mg of clarithromycin (CLR), 100 mg of fluconazole (FCZ), or 150 mg of rifabutin (RFB). The CLR study was conducted with 24 subjects. The geometric mean ratios (GMR) and 90% confidence intervals (90% CI; given in parentheses) of the areas under the concentration-time curve (AUC), the maximum concentrations of the drugs in serum (Cmax), and the concentrations in serum at 12 h postdose (Cp12h) for multiple-dose TPV/r and multiple-dose CLR, indicating the effect of TPV/r on the CLR parameters, were 1.19 (1.04-1.37), 0.95 (0.83-1.09), and 1.68 (1.42-1.98), respectively. The formation of the metabolite 14-OH-CLR was decreased by 95% in the presence of TPV, and the TPV AUC increased 66% compared to that for human immunodeficiency virus (HIV)-negative historical controls. The FCZ study was conducted with 20 subjects. The GMR (and 90% CI) of the AUC, Cmax, and Cp24h, indicating the effect of multiple-dose TPV/r on the multiple-dose FCZ parameters, were 0.92 (0.88-0.95), 0.94 (0.91-0.98), and 0.89 (0.85-0.92), respectively. The TPV AUC increased by 50% compared to that for HIV-negative historical controls. The RFB study was conducted with 24 subjects. The GMR (and 90% CI) of the AUC, Cmax, and Cp12h for multiple-dose TPV/r and single-dose RFB, indicating the effect of TPV/r on the RFB parameters, were 2.90 (2.59-3.26), 1.70 (1.49-1.94), and 2.14 (1.90-2.41), respectively. The GMR (and 90% CI) of the AUC, Cmax, and Cp12h of TPV/r and RFB with 25-O-desacetyl-RFB were 4.33 (3.86-4.86), 1.86 (1.63-2.12), and 2.76 (2.44-3.12), respectively. Coadministration of TPV with a single dose of RFB resulted in a 16% increase in the TPV Cp12h compared to that for TPV alone. In the general population, no dose adjustments are necessary for the combination of TPV/r and CLR or FCZ. Combining TPV/r with RFB should be done with caution, while toxicity and RFB drug levels should be monitored. Study medications were generally well-tolerated in these studies.
The treatment of human immunodeficiency virus (HIV)-infected patients with highly active antiretroviral therapy (HAART) has greatly improved life expectancy over the years (9). Nevertheless, opportunistic infections in people living with HIV continue to be a threat to the health of these individuals. Drug treatment of these opportunistic infections can be challenging because of the possible interactions between HAART and other drugs. This article presents the results of three studies of the interactions between tipranavir coadministered with low-dose ritonavir (TPV/r) and clarithromycin (CLR), fluconazole (FCZ), and rifabutin (RFB) in healthy adult volunteers.
Tipranavir (TPV) is an approved protease inhibitor (PI) with potent activity against PI-resistant HIV type 1 (HIV-1). TPV is highly plasma protein bound (99.98%) and is a substrate as well as an inducer of cytochrome P450 3A (CYP3A) (13; TPV product information [Boehringer Ingelheim International GmbH]). To achieve effective plasma TPV concentrations with a twice-daily (BID) dosing regimen in treatment-experienced patients, the coadministration of 500 mg of TPV and 200 mg of ritonavir is needed.
CLR is a macrolide antibacterial that is used extensively by people living with HIV infection or AIDS. In addition to providing multiple antibacterial effects, CLR may be used for both prophylaxis against and the treatment of Mycobacterium avium complex infections in AIDS patients. CLR is a substrate and an inhibitor of CYP3A. The primary metabolite is 14-hydroxy-R-clarithromycin (14-OH-CLR), which is the most active of the CLR metabolites (CLR product information; Abbott Laboratories).
FCZ is routinely indicated for oropharyngeal and esophageal candidiasis, as well as for the treatment of other serious systemic fungal infections in persons living with HIV infection. FCZ is cleared primarily by renal excretion, with a terminal elimination half-life (t1/2) of approximately 30 h. Approximately 80% of the administered dose appears in the urine as unchanged drug, and 11% of the dose is excreted in the urine as metabolites (FCZ product information; Pfizer, Inc.).
RFB is an antimycobacterial agent indicated for the prevention of disseminated M. avium complex disease in patients with advanced HIV infection or for the treatment of Mycobacterium tuberculosis infections. RFB is both an inducer and a substrate of CYP3A. CYP3A induction may decrease drug concentrations in plasma for drugs that are metabolized by the CYP3A enzyme system, while the inhibition of CYP3A may significantly increase the concentrations of RFB in plasma. Five RFB metabolites have been identified. The predominant metabolite, 25-O-desacetyl-rifabutin (25-O-desacetyl-RFB), has activity equal to that of the parent drug and contributes up to 10% to the total antimicrobial activity (RFB product information; Pharmacia & Upjohn).
The objective for these three drug interaction studies was to investigate the pharmacokinetic (PK) effects of TPV/r on the PKs of CLR, FCZ, and RFB and vice versa. The secondary objective for each study was to investigate the safety and tolerability of the study regimens.
MATERIALS AND METHODS
These studies were conducted with HIV-negative healthy male and female subjects. The study designs are summarized in Table 1.
TABLE 1.
CLR, FCZ, and RFB study designsa
| Study and procedure | Occurrence on study day:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| CLR study | |||||||||||||||||||||
| Blood sampling | P | P | P | ||||||||||||||||||
| TPV/r dosing | B | B | B | B | B | B | B | Q | |||||||||||||
| CLR dosing | B | B | B | B | B | B | B | B | B | B | B | B | Q | ||||||||
| FCZ study | |||||||||||||||||||||
| Blood sampling | P | P | P | P | P | ||||||||||||||||
| TPV/r dosing | B | B | B | B | B | B | B | ||||||||||||||
| FCZ dosing | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | ||||||||
| RFB study | |||||||||||||||||||||
| Blood sampling | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | ||||||
| TPV/r dosing | B | B | B | B | B | B | B | B | B | B | B | B | B | ||||||||
| RFB dosing | Q | Q | |||||||||||||||||||
P, PK blood sampling; B, BID dosing; Q, once-daily dosing. In all experiments, TPV/r was administered in a 500/200-mg dose. CLR was administered at 500 mg/dose, FCZ was administered at 200 mg on study day 1 and 100 mg on study days 2 to 13, and RFB was administered at 150 mg. The length of the CLR study was 13 days, that of the FCZ study was 14 days, and the RFB study lasted 21 days.
The studies were approved by an independent ethics committee, and all subjects gave written informed consent before any study-related procedure could take place. All three studies were conducted at MDS Pharma Services, St. Laurent, Quebec, Canada. The studies were conducted in compliance with the guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, the Declaration of Helsinki of 1996, and the Canadian Therapeutic Product Directorate. For each study, a detailed set of inclusion and exclusion criteria was defined. In general, male and female subjects (18 to 60 years of age) had to be nonsmokers in good health; have laboratory test values less than or equal to grade 1 values based on the Division of AIDS (DAIDS) grading scale; have an acceptable medical history, physical examination, and 12-lead echocardiogram results; have a healthy chest X-ray if deemed necessary by the investigator to establish the good health of the subject; and be able to give informed consent and adhere to the study protocol. Major exclusion criteria included serological evidence of hepatitis B virus, hepatitis C virus, and/or HIV infection, a seated systolic blood pressure of either <100 or >150 mm Hg, a resting heart rate of either <50 or >90 beats/min, pregnancy, breastfeeding, nonuse of barrier contraception for females, the use of hormonal contraception or hormone replacement therapy, recent participation in another drug trial, recent blood or plasma donations, the use of any other drugs, and alcohol or substance abuse.
Study design. (i) CLR interaction.
The subjects were given CLR at 500 mg BID from study day 1 until the morning of study day 13. BID administration of TPV/r in 500/200 mg doses was initiated on study day 6 and continued until the last dose on the morning of study day 13. Study drugs were taken with 240 ml of water on an empty stomach on PK study days and with a light snack on other days. Steady-state PK profiles for CLR were obtained on study day 5 (on which subjects received CLR alone) and day 13 (on which subjects received CLR and TPV/r), and the effect of first-dose TPV/r on CLR was evaluated from PK profiles obtained on day 6 (on which subjects received CLR and first-dose TPV/r). For the PK profiles, blood samples were obtained just before (nominal time, 0 h) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after drug intake in the morning. The effect of CLR on TPV in plasma was determined by comparing the TPV PK parameters on study day 13 to TPV data from a PK analysis of 68 healthy volunteers from four previous clinical studies (7, 13, 20, 24, 25). Ritonavir concentrations were not measured in this study.
(ii) FCZ interaction.
The subjects were given a loading dose of 200 mg of FCZ on study day 1, followed by a daily 100 mg FCZ dose for the remainder of the study. On study day 7, the subjects were started on a BID treatment regimen with TPV/r in a 500/200 mg dose. TPV/r treatment was continued until the last dose on the evening of study day 13. On study days 6, 7, and 13, PK plasma sampling was done just before (nominal time, 0 h) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 24 h after drug intake in the morning. Study drugs were taken with 240 ml of water on an empty stomach on PK study days and with a light snack on other days. Steady-state PK profiles for FCZ were obtained on study day 6 (on which subjects received FCZ only) and day 13 (on which subjects received FCZ and TPV/r), and the effect of first-dose TPV/r on FCZ was evaluated from PK profiles obtained on day 7 (on which subjects received FCZ and first-dose TPV/r). The effect of FCZ on TPV was determined by comparing the TPV PK parameters on study day 13 to TPV data from a PK analysis of 68 healthy volunteers from four previous clinical studies (7, 13, 20, 24, 25). Ritonavir concentrations were not measured in this study.
(iii) RFB interaction.
The subjects were given a single dose of RFB at 150 mg on study day 1. PK plasma sampling was done just before dosing (nominal time, 0 h) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48, 72, 96, 120, and 144 h after drug intake. On study day 8, subjects started TPV/r in a 500/200 mg dose BID and continued TPV/r treatment until study day 20. On study day 14, PK plasma sampling for TPV was done just before dosing (nominal time, 0 h) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after the morning drug intake. On study day 15, RFB was given as a single dose of 150 mg in the morning with the TPV/r dose and PK plasma sampling was done up to 144 h after administration. Study drugs were taken with 240 ml of water on an empty stomach on PK study days and with a light snack on other days. The effect of steady-state TPV/r on the single-dose PKs of RFB and 25-O-desacetyl-RFB were determined by comparing the PK parameters of RFB and 25-O-desacetyl-RFB on study days 1 and 15. The effect of single-dose RFB on the steady-state PKs of TPV was determined by comparing the PK parameters of TPV on study days 14 and 15. Ritonavir concentrations were not measured in this study.
Bioanalysis. (i) CLR and 14-OH-CLR.
A liquid chromatography-tandem mass spectrometry (LC-MS-MS) method with online automated extraction for the determination of CLR and 14-OH-CLR levels in human plasma had been validated previously by MDS Pharma Services, Saint Laurent, Quebec, Canada. An aliquot of human plasma containing CLR and 14-OH-CLR was extracted using an online extraction procedure. The extracted samples were analyzed by LC-MS-MS. The calibration curves ranged from 10.0 to 4,011.5 ng/ml for CLR and from 4.00 to 2,000.43 ng/ml for 14-OH-CLR. Intraday and interday accuracy and precision, expressed as the percentages of error and variation, were ≤7.3 and ≤13.2% (intraday) and ≤6.4 and ≤ 6.0% (interday), respectively, for CLR. For 14-OH-CLR, these results were ≤6.5 and ≤6.3% (intraday) and ≤8.1 and ≤9.1% (interday).
(ii) FCZ.
Plasma samples were analyzed for FCZ at BASi Analytics, West Lafayette, IN, by a validated LC-MS-MS method. FCZ was extracted from human heparinized plasma treated with a 10% ammonium hydroxide solution by liquid-liquid extraction with methyl-tert-butyl ether. The supernatant was blown down to the point of dryness. The extract was reconstituted and injected into an LC-MS-MS system using a Symmetry C18 analytical column (50 by 4.6 mm; particle size, 3.5 μm) with isocratic elution from a methanol-formic acid mobile phase. The FCZ calibration curve ranged from 0.0100 to 10.0 μg/ml. Intraday and interday accuracy and precision values were ≤17.0 and ≤10.0% (intraday) and ≤4.3 and ≤6.9% (interday), respectively.
(iii) RFB and 25-O-desacetyl-RFB.
An LC-MS-MS assay for RFB and 25-O-desacetyl-RFB was developed and validated by PPD Development, Middleton, WI. Briefly, plasma samples were extracted in the following manner. Extraction solvent (ethyl acetate-hexane, 60:40) and ammonium hydroxide were added to a 100 μl sample aliquot, and the mixture was then fortified with 20 μl of an internal standard working solution. Samples were subjected to a vortex and centrifuged, and the organic layer was transferred into tubes containing a 100% butyl ether keeper solution. Samples were evaporated, the remaining residue was reconstituted with 500 μl of a mobile phase, and hexane was added. Samples were again subjected to a vortex and centrifuged, and the hexane layer was aspirated as waste. The remaining solution was injected. The calibration curves ranged from 2.00 to 800 ng/ml for RFB and 25-O-desacetyl-RFB. Intraday and interday accuracy and precision values were ≤8.3 and ≤6.8% (intraday) and ≤3.2 and ≤2.5% (interday), respectively, for RFB. For 25-O-desacetyl-RFB, these results were ≤5.6 and ≤5.0% (intraday) and ≤5.1 and ≤3.4% (interday).
(iv) TPV.
Plasma samples were analyzed for TPV with a validated LC-MS-MS method at BASi Analytics, West Lafayette, IN. TPV and the internal standard were extracted from human heparinized plasma by a two-step liquid-liquid extraction method that used an ethyl acetate-hexane mixture followed by a hexane wash. The analytes were separated and detected by an LC-MS-MS system that used a 2.0- by 30-mm Synergi Polar RP column with a formic acid-acetic acid-acetonitrile mobile phase. The high calibration curve ranged from 1,000 to 20,000 ng/ml. The low calibration curve ranged from 25.0 to 2,000 ng/ml. Intraday and interday accuracy and precision values were ≤4.9 and ≤2.4% (intraday) and ≤6.8 and ≤ 6.1% (interday), respectively, for the high range. For the low range, these results were ≤5.2 and ≤1.4% (intraday) and ≤6.3 and ≤7.8% (interday).
PK analysis.
Noncompartmental methods were used for PK analysis with WinNonlin Professional software, version 4.0 (Pharsight Corporation, Mountain View, CA). The highest observed concentration of a drug in plasma was defined as Cmax, with the corresponding sampling time defined as Tmax. The concentrations at 12 and 24 h postdose were defined as Cp12h and Cp24h. The elimination rate constant (λz) was determined by least-squares linear regression analysis (log concentration versus time) of the last datum points (n ≥ 3). The t1/2 was calculated by using the following equation: t1/2 = ln2/λz. The area under the concentration-time curve (AUC; from 0 h to ∞ [AUC0-∞] for RFB, from 0 to 24 h [AUC0-24] for FCZ, and AUC0-12 for TPV and CLR) was estimated using the linear-log trapezoidal rule (linear up/log down). The apparent oral clearance (CL/F, where F is bioavailability) was calculated as the dose/AUC ratio, and the volume of distribution (V) was calculated as follows: (CL/F)/λz.
For the RFB study, because 25-O-desacetyl-RFB has antimicrobial activity equal to that of the parent drug, the PK of the two compounds were addressed using the sum of the RFB and 25-O-desacetyl-RFB AUC0-∞, Cmax, and Cp12h values, in addition to the analyses of the parent and metabolite alone. Results were converted using micromolar equivalents (RFB molecular weight, 847.02, and 25-O-desacetyl-RFB molecular weight, 804.97).
AEs.
Subject safety was monitored by the assessment of all adverse events (AEs) at each visit, in addition to the laboratory assessment of safety parameters by hematology and chemistry analyses and tests of liver function parameters (aspartate transaminase [AST], alanine aminotransferase [ALT], alkaline phosphatase, and total bilirubin levels) and lipid parameters (triglyceride and cholesterol levels) at the initial screening and on various days throughout the studies.
Statistical analysis.
Statistical analysis was done with SAS (release 8.2; SAS Institute Inc., Cary, NC). For CLR, FCZ, RFB, and TPV, the PK parameters of AUC, Cmax, and Cp12h or Cp24h were calculated as geometric means. The ratios of the geometric means for the test regimen to those for the reference regimen were used to assess the drug interaction (26). The null hypothesis was that the ratio being tested lay either below the lower boundary of relevance (0.80) or above the upper boundary of relevance (1.25). The alternative hypothesis was that the ratio lay within the relevance boundaries. The null hypothesis was tested using the methodology of two one-sided tests, i.e., the null hypothesis was rejected in favor of the absence of a relevant interaction if the 90% confidence interval (90% CI) for the ratio was completely contained within the acceptance region of 0.80 to 1.25. The 90% CI for the ratio was derived by the exponentiation of the CI from the logarithmic scale.
For TPV coadministered with CLR or FCZ, SAS Proc Multtest was used to resample TPV AUC0-12, Cmax, and Cp12h PK results for healthy volunteers from four previous clinical studies (7, 13, 20, 24, 25) and from the respective drug interaction studies. Bootstrap arithmetic means (2,000 resamples) were determined, and the ratios of the means for the test regimen to those for the reference regimen were used to assess the interaction and determine the point estimate. The 5th and 95th percentiles of the distribution of the ratios provided the 90% CIs.
The sample size for the CLR and RFB studies was based on TPV data that were on file. This approach was taken because the within-subject variation in the AUC for CLR was similar to that for TPV but, for RFB, no within-subject variation data were available; however, the between-subject variation was similar to that for TPV. Based on these data, a sample group of 18 subjects was required to ensure that the studies would have 90% power to reject both the null hypothesis that the ratio of the test mean to the standard mean was below 80% and the null hypothesis that the ratio of test mean to the standard mean was above 125%, i.e., that the test and standard were not equivalent, in favor of the alternative hypothesis that the means of the two groups were equivalent, assuming that the expected ratio of the means was 100%, that the coefficient of variation for the standard was 0.133, and that the data would be analyzed on the log scale at the 5% level. To ensure a sufficient sample size for analysis and allow for dropouts, 24 subjects were recruited and entered into both studies.
For the FCZ study, data from a previous study done by Boehringer Ingelheim were used to calculate the FCZ intraindividual coefficient of variation. The FCZ Cmax had a higher coefficient of variation than the other FCZ parameters and was used for the sample size calculation. For an expected reduction of PK parameters for FCZ of 5%, a sample size of 14 subjects was expected to result in a probability of 92% (power) that a 90% CI would be included in the acceptance range of 80 to 125%. To allow for dropouts, it was decided that 20 subjects would be included in the study.
RESULTS
CLR study.
Twenty-four healthy volunteers (7 females and 17 males) were enrolled in and completed the study. Two subjects were black, and 22 were white. The means ± standard deviations (SD) of age, weight, and height for the study population were 33.2 ± 9.2 years, 75.3 ± 12.3 kg, and 173.5 ± 10.5 cm, respectively.
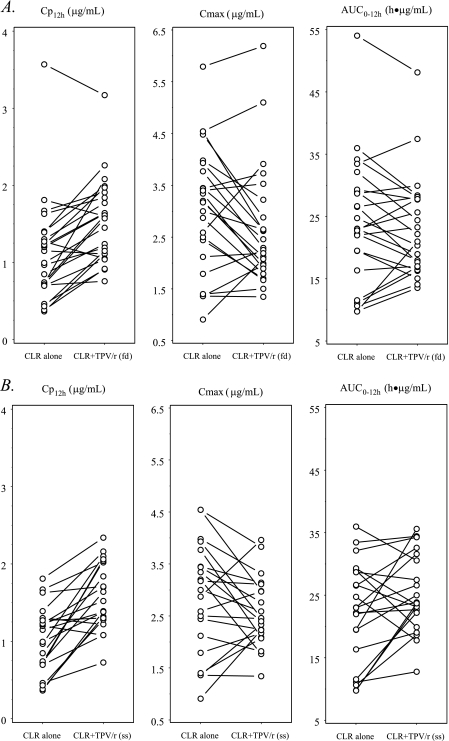
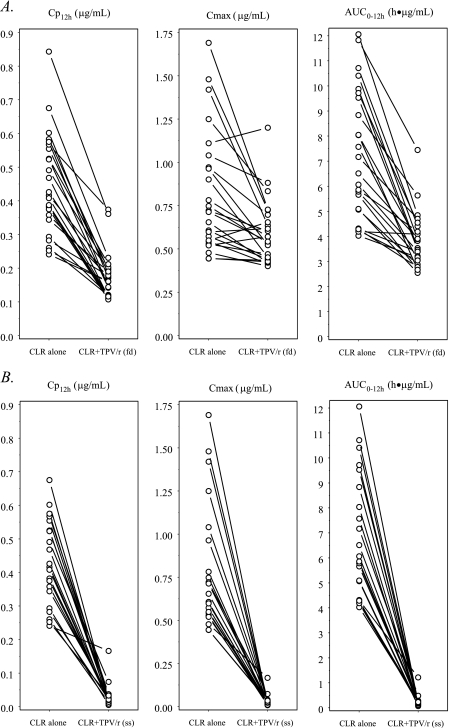
PK results for study days 10 and 13 were based on data from 21 subjects, due to recurrent vomiting by 3 subjects. Table 2 summarizes the geometric mean ratios and 90% CIs of the AUC0-12, Cmax, and Cp12h values for CLR and 14-OH-CLR in the presence and absence of single-dose and multiple-dose TPV (comparisons were made between data from the different study days). Table 3 summarizes the observed values for the main PK parameters of the studied compounds on the different study days. Figures 1 and 2 illustrate the individual AUC0-12, Cmax, and Cp12h values for CLR and 14-OH-CLR in the 21 subjects before and after the addition of TPV/r to the regimen. The addition of a single dose of TPV/r to the CLR regimen resulted in a 50% increase in the CLR Cp12h, and the addition of multiple-dose TPV/r resulted in a 68% increase in the CLR Cp12h. The Cp12h for the metabolite of CLR, 14-OH-CLR, was decreased by 61 and 95% after single and multiple doses of TPV/r, respectively. Multiple doses of TPV/r also resulted in a 97% decrease in both the AUC0-12 and Cmax for 14-OH-CLR. When steady-state CLR at 500 mg BID was coadministered with TPV/r in a 500/200-mg dose BID, the TPV AUC0-12, Cmax, and Cp12h increased 66, 40, and 100%, respectively, compared to the values for a healthy volunteer historical control population (Table 2).
TABLE 2.
Summary of mean ratios and 90% CIs for CLR, 14-OH-CLR, and TPV parameters
| Drug | Comparisona | No. of subjects | Mean ratio (90% CI) for:
|
||
|---|---|---|---|---|---|
| AUC0-12 | Cmax | Cp12h | |||
| CLR | CLR + sd TPV/r (day 6) vs CLR alone (day 5) | 24 | 1.00 (0.91-1.11) | 0.88 (0.78-1.00) | 1.50 (1.31-1.71) |
| CLR + md TPV/r (day 13) vs CLR alone (day 5) | 21 | 1.19 (1.04-1.37) | 0.95 (0.83-1.09) | 1.68 (1.42-1.98) | |
| 14-OH-CLR | 14-OH-CLR after CLR + sd TPV/r (day 6) vs CLR alone (day 5) | 24 | 0.54 (0.48-0.59) | 0.75 (0.68-0.83) | 0.39 (0.35-0.44) |
| 14-OH-CLR after CLR + md TPV/r (day 13) vs CLR alone (day 5) | 21 | 0.03 (0.02-0.04) | 0.03 (0.02-0.04) | 0.05 (0.04-0.07) | |
| TPV | CLR + md TPV/r (day 13) vs historical controls | 24 (68b) | 1.66 (1.43-1.73) | 1.40 (1.24-1.47) | 2.00 (1.58-2.47) |
sd, single-dose; md, multiple-dose. Unless otherwise noted, comparisons are between values for the same subjects on different study days, as indicated in parentheses.
Historical controls (n = 68).
TABLE 3.
Summary of the steady-state PK parameters of CLR and TPV
| PK parametera | Geometric mean (median, range)b for CLR in regimen of:
|
Geometric mean (median, range) for TPV in regimen of TPV/r + CLR (day 13) (n = 21) | ||
|---|---|---|---|---|
| CLR alone (day 5) (n = 24) | CLR + sd TPV/r (day 6) (n = 24) | CLR + md TPV/r (day 13) (n = 21) | ||
| AUC | 21.9 (23.0, 9.8-54.0) | 22.0 (21.6, 13.5-48.2) | 24.4 (23.7, 12.8-35.6) | 1,360 (1,367, 814-2,798) |
| Cmax | 2.80 (3.19, 0.91-5.79) | 2.47 (2.27, 1.35-6.19) | 2.49 (2.45, 1.34-3.96) | 176.9 (169.4, 110.4-361.3) |
| Cmin | 0.97 (1.07, 0.36-3.57) | 1.46 (1.59, 0.76-3.17) | 1.53 (1.52, 0.73-2.34) | 61.7 (68.1, 26.7-163.7) |
| Tmax (h) | 1.8c (1.5, 0.0-4.0) | 2.1 (1.5, 0.5-8.0) | 2.0 (2.0, 0.5-4.0) | 2.2 (2.0, 1.5-3.0) |
| t1/2 (h) | 6.1 (5.6, 4.1-16.2) | 10.2 (9.6, 3.7-21.3) | 11.3 (10.0, 6.5-20.8) | 6.4 (6.1, 2.6-17.9) |
| CL/F (liters/h) | 22.9 (21.7, 9.3-51.4) | 22.7 (23.2, 10.4-37.0) | 20.5 (21.1, 14.0-39.2) | 0.61 (0.61, 0.30-1.02) |
| V (liters) | 201 (180, 107-1,203) | 335 (347, 158-1,089) | 334 (319, 168-685) | 5.6 (5.9, 3.0-10.7) |
Unless otherwise indicated, parameters and units of measurement are as follows: for CLR, AUC0-12 (μg·h/ml), Cmax (μg/ml), and Cp12h (Cmin; μg/ml), and for TPV, AUC0-12 (μM·h), Cmax (μM), and Cp12h (Cmin; μM).
sd, single-dose; md, multiple-dose.
Excludes data for one subject with a Tmax of 0 h.
FIG. 1.
Effects of first-dose (fd) (A) and steady-state (ss) (B) TPV/r on the PKs of steady-state CLR. The symbols and lines represent data for individual subjects (fd, n = 24; ss, n = 21).
FIG. 2.
Effects of first-dose (fd) (A) and steady-state (ss) (B) TPV/r on the PKs of steady-state 14-OH-CLR. The symbols and lines represent data for individual subjects (fd, n = 24; ss, n = 21).
No serious AEs occurred during this study. There were no discontinuations due to AEs or any other reason. The most frequently reported AEs during CLR-TPV/r treatment included nausea (in 37.5% of subjects), loose stools (25%), headaches (25%), abdominal pain (16.7%), taste perversion (16.7%), vomiting (16.7%), and dizziness (12.5%). Most AEs were mild to moderate in intensity, with the majority (those in 54.2% of subjects) in the mild class. With the exception of one subject having a grade 3 ALT level during the CLR-TPV/r phase and one subject having a grade 3 lipase elevation during the CLR-only phase, there were no clinically relevant laboratory abnormalities.
FCZ study.
Twenty healthy volunteers (2 females and 18 males) were enrolled in and completed the study. One subject was black, and 19 were white. The means ± SD of age, weight, and height for the study population were 42.4 ± 11.0 years, 75.9 ± 9.5 kg, and 175.7 ± 6.8 cm, respectively.
PK results for FCZ were available for 19 subjects; the results for 1 subject were omitted because of an anomalous Cp24h for FCZ on study day 6. Table 4 summarizes the geometric mean ratios and 90% CIs of the AUC0-24, Cmax, and Cp24h values for FCZ in the comparisons made between data from the different study days. Table 5 summarizes the observed values for the main PK parameters for FCZ and TPV on the different study days. Figure 3 illustrates the individual AUC, Cmax, and Cp24h values for FCZ in the 19 subjects, before and after the addition of TPV/r to the regimen.
TABLE 4.
Summary of mean ratios and 90% CIs for FCZ and TPV
| Drug | Comparisona | No. of subjects | Mean ratiob (90% CI) for:
|
||
|---|---|---|---|---|---|
| AUC | Cmax | Cmin | |||
| FCZ | FCZ alone (day 6) vs FCZ + sd TPV/r (day 7) | 19 | 0.99 (0.97-1.02) | 0.97 (0.94-1.01) | 0.98 (0.94-1.02) |
| FCZ alone (day 6) vs FCZ + md TPV/r (day 13) | 19 | 0.92 (0.88-0.95) | 0.94 (0.91-0.98) | 0.89 (0.85-0.92) | |
| TPV | FCZ + md TPV/r (day 13) vs historical controls | 20 (68c) | 1.50 (1.29-1.73) | 1.32 (1.18-1.47) | 1.69 (1.33-2.09) |
sd, single-dose; md, multiple-dose. Unless otherwise noted, comparisons are between values for the same subjects on different study days, as indicated in parentheses.
Parameters are AUC0-24 and Cp24h (Cmin) for FCZ and AUC0-12 and Cp12h (Cmin) for TPV.
Historical controls (n = 68).
TABLE 5.
Summary of the steady-state PK parameters of FCZ and TPV
| PK parametera | Geometric mean (median, range) for FCZ in regimen of:
|
Geometric mean (median, range) for TPV in regimen of TPV/r + FCZ (day 13) (n = 20) | ||
|---|---|---|---|---|
| FCZ alone (day 6) (n = 19) | FCZ + 2 doses of TPV/r (day 7) (n = 19) | FCZ + steady-state TPV/r (day 13) (n = 19) | ||
| AUC | 99.0 (97.1, 81.7-149.8) | 98.2 (93.4, 79.5-136.5) | 90.6 (90.2, 71.5-145.7) | 1,333 (1,307, 708-2674) |
| Cmax | 5.3 (4.9, 4.4-8.6) | 5.1 (4.9, 4.0-7.0) | 4.9 (4.7, 3.9-8.0) | 179.9 (173.1, 126.1-277.9) |
| Cmin | 3.6 (3.5, 2.8-5.4) | 3.5 (3.3, 2.8-5.0) | 3.2 (3.2, 2.3-4.9) | 59.5 (59.0, 26.1-156.9) |
| Tmax (h) | 2.1 (2.0, 1.0-4.0) | 2.8 (3.0, 1.5-10.0) | 2.4 (3.0, 1.5-4.0) | 2.3 (2.0, 1.5-4.0) |
| t1/2 (h) | 50.8 (50.2, 30.6-74.1) | 46.9 (44.0, 26.2-77.7) | 46.0 (45.2, 31.6-74.3) | 6.3 (6.4, 3.1-15.6) |
| CL/F (liters/h) | 1.0 (1.0, 0.7-1.2) | 1.0 (1.1, 0.7-1.3) | 1.1 (1.1, 0.7-1.4) | 0.62 (0.63, 0.31-1.17) |
| V (liters) | 74.0 (72.9, 50.7-121.0) | 68.9 (66.9, 38.8-108.4) | 73.2 (71.1, 41.0-136.5) | 5.6 (5.6, 2.8-8.5) |
Unless otherwise indicated, parameters and units of measurement are as follows: for FCZ, AUC0-24 (μg·h/ml), Cmax (μg/ml), and Cp24h (Cmin; μg/ml), and for TPV, AUC0-12 (μM·h), Cmax (μM), and Cp12h (Cmin; μM).
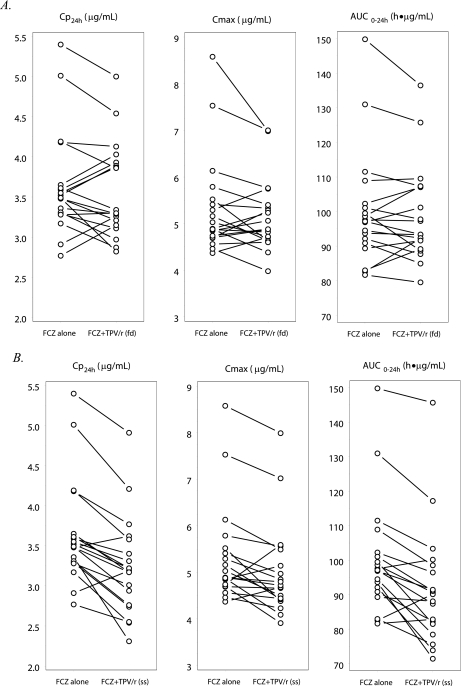
FIG. 3.
Effects of first-dose (fd) (A) and steady-state (ss) (B) TPV/r on the PKs of steady-state FCZ. The symbols and lines represent data for individual subjects (n = 19).
The effect of a single 500/200-mg dose of TPV/r on the FCZ AUC, Cmax, and Cp24h was minimal (change, <3%) (Table 4). After multiple doses of TPV/r, the decrease in FCZ exposure was <12%. In both dosing situations, the 90% CIs for the geometric mean ratios were within the bioequivalence limits of 0.80 to 1.25.
When steady-state FCZ at 100 mg once daily was coadministered with TPV/r in a 500/200-mg dose BID, the TPV AUC0-12, Cmax, and Cp12h increased 50, 32, and 69%, respectively, compared to those for the healthy volunteer historical control population (Table 4).
No serious AEs occurred during this study. There were no discontinuations due to AEs or any other reason. The most frequently observed AEs were gastrointestinal tract related (loose stool [in 35% of subjects], nausea [20%], and lower abdominal pain [15%]). Dizziness (excluding vertigo, which was not observed in this study) and somnolence each occurred in 20% of subjects. The majority of these events were of mild to moderate intensity, with 16 subjects (80%) having mild events and 2 subjects (10%) having moderate events. There were no clinically relevant variations from the baseline in the results of laboratory tests during the coadministration of TPV/r and FCZ. Relative to treatment with FCZ alone, treatment with TPV/r plus FCZ resulted in a median 2.1-fold increase in ALT levels and a 1.5-fold increase in AST levels; however, no adverse clinical symptoms or events were associated. There were no relevant differences in the medians of other liver function test parameters between treatments with FCZ alone and with TPV/r plus FCZ.
RFB study.
Twenty-four healthy volunteers (4 females and 20 males) were enrolled in and 20 completed the study; 4 subjects discontinued participation due to AEs. Twenty-two subjects were white, and two were black. The means ± SD of age, weight, and height for the study population were 32.8 ± 8.5 years, 73.2 ± 8.9 kg, and 173.8 ± 8.6 cm, respectively.
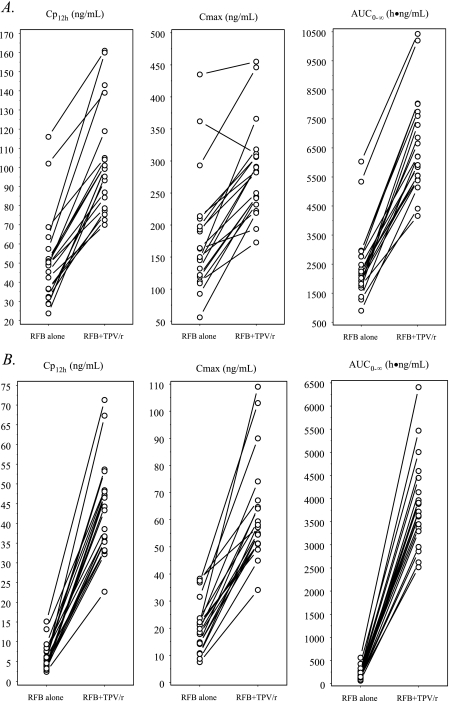
Three subjects withdrew from the study prior to study day 15, and one subject withdrew from the study on study day 16. Therefore, PK results for RFB and 25-O-desacetyl-RFB were available for 20 subjects, while PK results for TPV were available for 21 subjects. Table 6 summarizes the geometric mean ratios and 90% CIs of the AUC0-∞, Cmax, and Cp12h values for TPV, RFB, and 25-O-desacetyl-RFB and the sum of the values for RFB and 25-O-desacetyl-RFB in the comparisons made between data from the different study days. Figure 4 illustrates the individual AUC, Cmax, and Cp12 values for RFB and its metabolite in the 20 subjects, with and without TPV/r. Table 7 summarizes the observed values of the main PK parameters for TPV, RFB, and 25-O-desacetyl-RFB on the different study days. The coadministration of steady-state TPV/r and single-dose RFB caused statistically significant increases in all three primary PK parameters (AUC0-∞, Cmax, and Cp12h) for both the parent drug (RFB) and the active metabolite (25-O-desacetyl-RFB).
TABLE 6.
Summary of mean ratios and 90% CIs for RFB, 25-O-desacetyl-RFB (metabolite), and the total of the parent plus the metabolite, as well as TPV
| Drug(s) | Comparisona | No. of subjects | Mean ratiob (90% CI) for:
|
||
|---|---|---|---|---|---|
| AUC | Cmax | Cmin | |||
| RFB | sd RFB alone (day 1) vs sd RFB + md TPV/r (day 15) | 20 | 2.90 (2.59-3.26) | 1.70 (1.49-1.94) | 2.14 (1.90-2.41) |
| RFB metabolite | sd RFB alone (day 1) vs sd RFB + md TPV/r (day 15) | 20 | 20.71 (17.66-24.28) | 3.20 (2.78-3.68) | 7.83 (6.70-9.14) |
| RFB and metabolite | sd RFB alone (day 1) vs sd RFB + md TPV/r (day 15) | 20 | 4.33 (3.86-4.86) | 1.86 (1.63-2.12) | 2.76 (2.44-3.12) |
| TPV | TPV/r alone (day 14) vs TPV/r + sd RFB (day 15) | 21 | 1.00 (0.96-1.04) | 0.99 (0.93-1.07) | 1.16 (1.07-1.27) |
sd, single-dose; md, multiple-dose. Comparisons are between values for the same subjects on different study days, as indicated in parentheses.
Parameters are AUC0-∞ for RFB, AUC0-12 for TPV, and Cp12h (Cmin) for RFB and TPV.
FIG. 4.
Effects of steady-state TPV/r on the PKs of single-dose RFB (A) and 25-O-desacetyl-RFB (B). The symbols and lines represent data for individual subjects (n = 20).
TABLE 7.
Summary of the steady-state PK parameters of TPV, RFB, and 25-O-desacetyl-RFBa
| PK parameterb | Result for TPV in regimen of:
|
Result for RFB in regimen of:
|
Result for 25-O-desacetyl-RFB in regimen of:
|
|||
|---|---|---|---|---|---|---|
| TPV/r alone (day 14) (n = 21) | TPV/r + RFB (day 15) (n = 21) | RFB alone (day 1) (n = 20) | RFB + TPV/r (day 15) (n = 20) | RFB alone (day 1) (n = 20) | RFB + TPV/r (day 15) (n = 20) | |
| AUC | 955 (980, 537-1,444) | 953 (996, 586-1,761) | 2,217 (2,157, 895-6,032) | 6,441 (6,206, 4,160-10,426) | 182 (175, 64-560) | 3,775 (3,716, 2,518-6405) |
| Cmax | 140.6 (141.1, 84.5-213.1) | 139.7 (143.7, 87.0-284.7) | 162.0 (156.5, 55.9-435.0) | 275.5 (283.0, 173.0-455.0) | 18.7 (20.4, 7.5-38.3) | 59.8 (56.1, 34.1-109.0) |
| Cmin | 35.2 (38.8, 10.5-79.2) | 41.0 (41.4, 16.5-97.6) | 46.7 (49.0, 23.7-116.0) | 100.1 (99.3, 70.0-161.0) | 5.4 (4.9, 2.4-15.1) | 42.2 (43.8, 22.7-71.3) |
| Tmax (h) | 2.8 (3.0, 2.0-4.0) | 2.6 (3.0, 1.5-4.0) | 3.5 (3.0, 2.0-6.0) | 4.1 (4.0, 2.0-6.0) | 3.6 (3.0, 2.0-6.0) | 5.8 (6.0, 4.0-8.0) |
| t1/2 (h) | 4.7 (4.5, 2.5-8.0) | 5.5 (5.8, 3.0-11.1) | 41.3 (51.8, 11.7-84.8) | 65.1 (71.2, 42.5-91.6) | 6.5 (6.1, 3.5-14.1) | 64.2 (68.9, 37.3-84.6) |
| CL/F (liters/h) | 0.87 (0.85, 0.57-1.54) | 0.87 (0.47, 0.83-1.42) | 67.7 (69.6, 24.9-167.6) | 23.3 (24.2, 14.4-36.1) | NA | NA |
| V (liters) | 5.9 (6.1, 3.6-9.1) | 7.0 (7.3, 3.6-9.8) | 4,028 (4,368, 1,542-8,638) | 2,188 (2,318, 909-3205) | NA | NA |
Data are given as the geometric mean (median, range). NA, not applicable.
Unless otherwise indicated, parameters and units of measurement are as follows: for RFB, AUC0-∞ (ng·h/ml), Cmax (ng/ml), and Cp12h (Cmin; ng/ml); and for TPV, AUC0-12 (μM·h), Cmax (μM), and Cp12h (Cmin; μM).
The coadministration of single-dose RFB and steady-state TPV/r did not affect the primary TPV PK parameters of AUC0-12 or Cmax and caused only a small, but statistically significant, 16% increase in the Cp12h.
Eighteen subjects (75%) reported at least one AE while receiving TPV/r. The most frequently reported AEs were related to the gastrointestinal tract (those reported by 14 subjects [58.4%], including nausea [33.3%] and vomiting [25%]) and the central nervous system (those reported by 11 subjects [45.8%], including headaches [33.3%] and dizziness [12.5%]). All AEs during treatment with TPV/r were of mild to moderate severity according to DAIDS grading, with 18 subjects (75%) reporting mild events and 3 subjects (12.5%) reporting moderate events. Five subjects were found to have clinically relevant laboratory test abnormalities. Three subjects developed asymptomatic levels of ALT or AST greater than or equal to Division of AIDS grade 3 levels, which led to their early discontinuation from the study. In all 3 subjects, ALT and AST levels returned to normal by a follow-up visit approximately 1 month following the end of the study. A grade 3 elevation in the lipase level (on day 21) and a grade 4 elevation in the partial thromboplastin time level (on day 8) were also reported during the study; these levels returned to normal 1 week later.
A fourth subject discontinued participation due to the appearance of a generalized rash, which resolved after 5 days. All the AEs that led to discontinuations occurred during the TPV/r treatment period.
DISCUSSION
This article presents the results of three healthy-volunteer studies which investigated the PK interaction between TPV/r and each of the antimicrobial drugs CLR, FCZ, and RFB. These drug combinations are frequently needed in the treatment of HIV-infected patients, and therefore, PK drug-drug interaction studies of these combinations were warranted.
CLR study PKs.
In the CLR study, it was found that the CLR AUC and Cp12h increased 19 and 68% and that the Cmax decreased 5% after coadministration with steady-state TPV/r. The effect of TPV/r on 14-OH-CLR was greater and almost completely (>95%) inhibited the formation of this metabolite. These observations are consistent with previously published results with other PIs. In an interaction study with ritonavir at 200 mg three times a day (TID) and CLR at 500 mg BID, increases in the CLR AUC, Cmax, and Cmin of 77, 31, and 182%, respectively, were seen. Also in this study, the formation of 14-OH-CLR was strongly inhibited (>99%) (15). In an interaction study with indinavir at 800 mg TID and CLR at 500 mg BID, it was found that the CLR AUC and Cmax increased by 47 and 19%, respectively, while the 14-OH-CLR AUC and Cmax decreased by 49 and 48% (2). The combination of amprenavir at 1,200 mg BID and CLR at 500 mg BID had only a small effect (<10%) on the CLR AUC, Cmax, and Cp12h; however, for 14-OH-CLR, these parameters were decreased by 35, 32, and 4%, respectively (3). In that study, it was noticed that the renal clearance of CLR increased 34% when CLR was combined with amprenavir. This observation can explain the relatively small increase in the CLR AUC for this combination. Similarly, in the present study, the relatively small increase of 19% in the CLR AUC may be the result of increased renal clearance. The changes in CLR PKs when CLR is combined with TPV/r normally will not require a dose adjustment for CLR, given the wide therapeutic range of CLR levels. However, when renal function is decreased, a decrease in the CLR dose will likely be necessary to prevent supratherapeutic drug concentrations (CLR product information; Abbott Laboratories).
The increase in TPV exposure of 66%, in comparison to that of the healthy volunteer historical controls, can be explained by the inhibitory effects of CLR on CYP3A isozymes. For amprenavir, indinavir, and ritonavir, increases in the AUCs of 18, 19, and 12%, respectively, occur when these drugs are combined with CLR (2, 3, 15).
FCZ study PKs.
In the FCZ study, the coadministration of FCZ and two 500/200-mg doses of TPV/r did not substantially influence the steady-state PKs of FCZ (changes in the AUC0-24, Cmax, and Cp24h were ≤3%). The coadministration of FCZ and TPV/r in a 500/200-mg dose BID for 7 days caused small, but statistically significant, decreases in the FCZ AUC0-24 (−8%), Cmax (−6%), and Cp24h (−11%). However, these minor changes are not considered to be clinically significant. The absence of a clinically relevant effect of TPV/r on FCZ PKs can be explained by the fact that FCZ is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites. FCZ has only weak affinity for human cytochrome P450 metabolic enzymes. However, the increases in the steady-state AUC0-12, Cmax, and Cp12h of TPV, compared to the data for the healthy volunteer historical controls, may be explained by the inhibitory effect of FCZ on metabolism mediation by CYP3A (5, 12), the main enzyme for the metabolism of both TPV and ritonavir.
Similarly, FCZ (at different dosages) has been shown to influence concentrations of other HIV PIs in plasma. FCZ (200 mg once daily) increased the AUC0-24 and Cmax of ritonavir (200 mg every 6 h) by 12 and 15%, respectively, in a study with eight healthy volunteers (5). The AUC0-8 and Cmax of saquinavir (1,200 mg TID) were increased by 50 and 56%, respectively, during the coadministration of FCZ (200 mg once daily) in a small study with five HIV-1-infected patients (12). In the same study, no effect of FCZ on the PKs of ritonavir (600 mg BID) was observed (n = 3). In a study with 11 HIV-1-infected patients, the coadministration of FCZ (400 mg once daily) and indinavir (1,000 mg TID) caused a marginally statistically significant decrease in the AUC0-8 of indinavir (−24%), with no substantial effect on the Cmax or Cp8h (8). The reduction of the indinavir AUC may be explained by the potential interference of FCZ with indinavir absorption or the induction of certain cytochrome P450 enzymes by FCZ.
An increase in the Cmax (+32%) and a decrease in the CL/F (−36%) of TPV compared to the PK results for the historical control population were observed during the coadministration of TPV/r and FCZ, suggesting that FCZ inhibited both intestinal and hepatic CYP3A enzymes. This finding is in agreement with the results of a previously published PK interaction study of oral FCZ and intravenous and oral midazolam, which is a well-known substrate for CYP3A (14).
RFB study PKs.
In the RFB study, the effect of a single 150-mg dose of RFB on the steady-state PKs of TPV (coadministered with ritonavir) was limited to a 16% increase in the Cp12h, with no apparent effect on the AUC0-12 or Cmax. This outcome is in line with the knowledge that RFB-mediated CYP3A induction requires approximately 1 week of daily drug administration (4, 17). Therefore, an RFB-dependent CYP3A induction effect was unlikely to occur since this study was limited to investigating the interactions between a single dose of RFB and steady-state TPV/r. However, from the findings in several reports, it seems that multiple doses of RFB in healthy volunteers may lead to unacceptable AEs (1, 10, 23).
When a single 150-mg dose of RFB was coadministered with TPV/r, the RFB AUC0-∞, Cmax, and Cp12h increased 2.90-, 1.70-, and 2.14-fold, respectively. Greater changes were observed for 25-O-desacetyl-RFB, with the AUC0-∞, Cmax, and Cp12h increasing 20.71-, 3.20-, and 7.83-fold, respectively, and the combined parent-metabolite AUC0-∞, Cmax, and Cp12h increasing 4.33-, 1.86-, and 2.76-fold.
The greater effect of TPV/r on the PKs of 25-O-desacetyl-RFB than on those of RFB can be explained by the differential contribution of CYP3A to the metabolism of RFB and that of 25-O-desacetyl-RFB, as proposed previously (6). The changes in the PKs of RFB and 25-O-desacetyl-RFB and the metabolite/parent ratio in the presence of TPV/r are likely the outcome of the potent inhibitory effect that ritonavir exerts on CYP3A activity. This inhibition of CYP3A can occur in both the intestinal wall and the liver, resulting in decreased first-pass metabolism at both sites (6). The results of the present study are consistent with previously observed increases in RFB and 25-O-desacetyl-RFB in healthy volunteers receiving RFB (150 mg once a day for 16 days) coadministered with ritonavir (500 mg every 12 h for 10 days) at steady state (6). In the latter study, RFB and 25-O-desacetyl-RFB AUCs increased approximately 4- and 35-fold, respectively, when the drugs were coadministered with ritonavir. The combined parent-metabolite AUC increased nearly sevenfold. Also, the ratio of the metabolite to the parent increased by approximately 77%, which compares well with the 65% increase in the present study.
The results of a clinical PK study with HIV-positive patients without mycobacterial infection who were receiving saquinavir at 400 mg and ritonavir at 400 mg demonstrated that RFB dosing every 3 days at 150 mg produces peak and trough concentrations that are similar to those produced by the 300-mg daily RFB dose in the absence of CYP3A inhibitors (11).
To manage the clinically important changes in the PKs of RFB and 25-O-desacty-RFB when combined with TPV/r, therapeutic drug monitoring (TDM) of RFB may be useful. The expected large interpatient variability in these interactions increases the need for TDM of RFB to determine the best dose adjustment for the individual patient. TDM of RFB, including cutoff values, has been described in more detail in the literature (16, 21, 22). Finally, patients receiving RFB with TPV/r should be closely monitored for the emergence of AEs associated with RFB therapy.
Safety.
In terms of safety, these studies were conducted with healthy male and female subjects between 18 and 60 years of age. Consistent with the results of previous TPV trials, the most frequently observed AEs were gastrointestinal tract related. In general, the treatments in the CLR and FCZ studies were well-tolerated, with the majority of AEs’ being mild in intensity. The clinically nonrelevant increases in ALT and AST in the FCZ study seem to be concordant with a report of liver toxicity's being related to FCZ use in tuberculosis treatment (18).
In the RFB study, five subjects were found to have clinically relevant changes in laboratory measurements. Three subjects developed asymptomatic grade 3 and 4 levels of ALT and AST, which led to their early discontinuation from the study. A fourth subject discontinued participation due to the appearance of a generalized rash. All the AEs that led to discontinuations occurred during the TPV/r treatment period. Overall, the RFB and TPV/r combination treatment was moderately tolerated.
Conclusion.
In summary, when combining TPV with CLR, it should be noted that both TPV and CLR exposure will be increased. For CLR, this will not cause problems in patients with normal renal function. However, while concentration-related AEs have not been observed in studies with TPV (19), patients using CLR at doses higher than 500 mg BID should be carefully monitored for signs of toxicity. The almost complete inhibition of the formation of 14-OH-CLR should be taken into account when treating pathogens susceptible to this pharmacologically active metabolite. Overall, the TPV/r-CLR combination treatment was moderately tolerated in the healthy subject population used in this study; however, it is not clear if this is a result of the increase in CLR exposure.
The coadministration of FCZ (100 mg once daily) and TPV/r in a 500/200-mg dose BID does not have a clinically relevant effect on the PKs of FCZ. Therefore, no dosage adjustment is required for FCZ when combined with TPV/r in a 500/200-mg dose. FCZ appeared to have a significant effect on the steady-state PKs of TPV compared to the PKs of TPV/r alone in HIV-negative individuals. The clinical significance of this increase in the exposure to TPV is not known, but it was previously shown that a 45.6% increase in TPV exposure does not result in increased toxicity (19); however, clinical monitoring of patients receiving this combination is advised. No unexpected safety issues arose in this study, and medications were well-tolerated in these healthy volunteers.
The effect of a single 150-mg dose of RFB on the steady-state PKs of TPV resulted in an average 16% increase in the Cp12h, with no effect on the AUC0-12 or Cmax occurring. This small increase in the trough TPV concentration does not appear to be clinically relevant. As a result of CYP3A inhibition by ritonavir, changes of clinical importance in the PKs of RFB and its active metabolite, 25-O-desacetyl-RFB, occurred. Considering the AUCs of the parent and metabolite together revealed a >4-fold increase in exposure after the administration of RFB at 150 mg. When RFB is coadministered with TPV/r, RFB drug levels should be monitored by TDM and the dose should be adjusted accordingly. In addition, patients receiving RFB with TPV/r should be closely monitored for RFB toxicity by clinical judgment and laboratory assessments.
Acknowledgments
We thank the healthy volunteers that participated in these studies.
These studies were supported by Boehringer Ingelheim Canada Ltd. C.L.P. has received grants or research support from or served as a consultant, advisor, or speaker for Abbott Laboratories, Bristol-Myers Squibb, Merck, Roche, Boehringer Ingelheim, Pfizer, and Tibotec. D.W.C. is supported by a career scientist award from the Ontario Ministry of Health (Ontario HIV Treatment Network). D.W.C. has received support from and contracted research through his institution (University of Ottawa at The Ottawa Hospital) for Boehringer Ingelheim, Pharmacia & Upjohn, and Abbott Laboratories.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Apseloff, G. 2003. Severe neutropenia among healthy volunteers given rifabutin in clinical trials. Clin. Pharmacol. Ther. 74:591-593. [DOI] [PubMed] [Google Scholar]
- 2.Boruchoff, S. E., M. G. Sturgill, K. W. Grasing, J. R. Seibold, J. McCrea, G. A. Winchell, S. E. Kusma, and P. J. Deutsch. 2000. The steady-state disposition of indinavir is not altered by the concomitant administration of clarithromycin. Clin. Pharmacol. Ther. 67:351-359. [DOI] [PubMed] [Google Scholar]
- 3.Brophy, D. F., D. S. Israel, A. Pastor, C. Gillotin, G. E. Chittick, W. T. Symonds, Y. Lou, B. M. Sadler, and R. E. Polk. 2000. Pharmacokinetic interaction between amprenavir and clarithromycin in healthy male volunteers. Antimicrob. Agents Chemother. 44:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burman, W. J., K. Gallicano, and C. Peloquin. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327-341. [DOI] [PubMed] [Google Scholar]
- 5.Cato, A., III, G. Cao, A. Hsu, J. Cavanaugh, J. Leonard, and R. Granneman. 1997. Evaluation of the effect of fluconazole on the pharmacokinetics of ritonavir. Drug Metab. Dispos. 25:1104-1106. [PubMed] [Google Scholar]
- 6.Cato, A., III, J. Cavanaugh, H. Shi, A. Hsu, J. Leonard, and R. Granneman. 1998. The effect of multiple doses of ritonavir on the pharmacokinetics of rifabutin. Clin. Pharmacol. Ther. 63:414-421. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, C., R. van Heeswijk, M. Bilodeau, B. Kovacs, J. Sabo, T. MacGregor, J. Wruck, M. Elgadi, D. Neubacher, and S. McCallister. 2005. The pharmacokinetics (PK) of single-dose and steady-state tipranavir/ritonavir (TPV/r) 500 mg/200 mg in subjects with mild or moderate hepatic impairment, abstr. 3.11. Sixth Int. Workshop Clin. Pharmacol. HIV Ther., Montreal, Quebec, Canada, 28 to 30 April 2005.
- 8.De Wit, S., M. Debier, M. De Smet, J. McCrea, J. Stone, A. Carides, C. Matthews, P. Deutsch, and N. Clumeck. 1998. Effect of fluconazole on indinavir pharmacokinetics in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 42:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger, M., M. May, G. Chene, A. N. Phillips, B. Ledergerber, F. Dabis, D. Costagliola, A. D'Arminio Monforte, F. de Wolf, P. Reiss, J. D. Lundgren, A. C. Justice, S. Staszewski, C. Leport, R. S. Hogg, C. A. Sabin, M. J. Gill, B. Salzberger, and J. A. Sterne. 2002. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360:119-129. [DOI] [PubMed] [Google Scholar]
- 10.Flexner, C., and P. Barditch-Crovo. 2003. Severe neutropenia among healthy volunteers given rifabutin in clinical trials. Clin. Pharmacol. Ther. 74:592-593. [DOI] [PubMed] [Google Scholar]
- 11.Gallicano, K., Y. Khaliq, G. Carignan, A. Tseng, S. Walmsley, and D. W. Cameron. 2001. A pharmacokinetic study of intermittent rifabutin dosing with a combination of ritonavir and saquinavir in patients infected with human immunodeficiency virus. Clin. Pharmacol. Ther. 70:149-158. [DOI] [PubMed] [Google Scholar]
- 12.Koks, C. H., K. M. Crommentuyn, R. M. Hoetelmans, D. M. Burger, P. P. Koopmans, R. A. Mathot, J. W. Mulder, P. L. Meenhorst, and J. H. Beijnen. 2001. The effect of fluconazole on ritonavir and saquinavir pharmacokinetics in HIV-1-infected individuals. Br. J. Clin. Pharmacol. 51:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGregor, T. R., J. P. Sabo, S. H. Norris, P. Johnson, L. Galitz, and S. McCallister. 2004. Pharmacokinetic characterization of different dose combinations of coadministered tipranavir and ritonavir in healthy volunteers. HIV Clin. Trials 5:371-382. [DOI] [PubMed] [Google Scholar]
- 14.Olkkola, K. T., J. Ahonen, and P. J. Neuvonen. 1996. The effects of the systemic antimycotics, itraconazole and fluconazole, on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Anesth. Analg. 82:511-516. [DOI] [PubMed] [Google Scholar]
- 15.Ouellet, D., A. Hsu, G. R. Granneman, G. Carlson, J. Cavanaugh, H. Guenther, and J. M. Leonard. 1998. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin. Pharmacol. Ther. 64:355-362. [DOI] [PubMed] [Google Scholar]
- 16.Peloquin, C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169-2183. [DOI] [PubMed] [Google Scholar]
- 17.Polk, R. E., D. F. Brophy, D. S. Israel, R. Patron, B. M. Sadler, G. E. Chittick, W. T. Symonds, Y. Lou, D. Kristoff, and D. S. Stein. 2001. Pharmacokinetic interaction between amprenavir and rifabutin or rifampin in healthy males. Antimicrob. Agents Chemother. 45:502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pukenyte, E., F. X. Lescure, D. Rey, C. Rabaud, B. Hoen, P. Chavanet, A. P. Laiskonis, J. L. Schmit, T. May, Y. Mouton, and Y. Yazdanpanah. 2007. Incidence of and risk factors for severe liver toxicity in HIV-infected patients on anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 11:78-84. [PubMed] [Google Scholar]
- 19.Raffi, F., M. Battegay, S. Rusconi, M. Opravil, G. Blick, R. T. Steigbigel, M. Kraft, D. Neubacher, and J. P. Sabo. 2007. Combined tipranavir and enfuvirtide use associated with higher plasma tipranavir concentrations but not with increased hepatotoxicity: sub-analysis from RESIST. AIDS 21:1977-1980. (Erratum, 22:i, 2008.) [DOI] [PubMed] [Google Scholar]
- 20.Sabo, J. P., C.-L. Yong, T. R. MacGregor, M. Castles, G. Mukwaya, V. Kohlbrenner, P. Robinson, and M. Kraft. 2007. Nonlinear mixed effects modeling of the steady-state pharmacokinetics of tipranavir for adult healthy volunteers and HIV+ patients receiving TPV 500 mg bid coadministered with RTV 100 mg or 200 mg bid, abstr. 7. Eighth Int. Workshop Clin. Pharmacol. HIV Ther., Budapest, Hungary.
- 21.Schwiesow, J. N., M. D. Iseman, and C. A. Peloquin. 2008. Concomitant use of voriconazole and rifabutin in a patient with multiple infections. Pharmacotherapy 28:1076-1080. [DOI] [PubMed] [Google Scholar]
- 22.Spradling, P., D. Drociuk, S. McLaughlin, L. M. Lee, C. A. Peloquin, K. Gallicano, C. Pozsik, I. Onorato, K. G. Castro, and R. Ridzon. 2002. Drug-drug interactions in inmates treated for human immunodeficiency virus and Mycobacterium tuberculosis infection or disease: an institutional tuberculosis outbreak. Clin. Infect. Dis. 35:1106-1112. [DOI] [PubMed] [Google Scholar]
- 23.Stein, C. M. 2003. Managing risk in healthy subjects participating in clinical research. Clin. Pharmacol. Ther. 74:511-512. [DOI] [PubMed] [Google Scholar]
- 24.van Heeswijk, R., J. P. Sabo, C. Cooper, W. Cameron, T. R. MacGregor, M. Elgadi, F. Harris, S. McCallister, and D. Mayers. 2004. The pharmacokinetic interaction between tipranavir/ritonavir 500 mg/200 mg bid (tipranavir/r) and atorvastatin, antacid and CYP3A4 in healthy volunteers, abstr. 5.2. Fifth Int. Workshop Clin. Pharmacol. HIV Ther., Rome, Italy.
- 25.van Heeswijk, R., J. Sabo, T. MacGregor, M. Elgadi, F. Harris, S. McCallister, and D. Mayers. 2004. The pharmacokinetic interaction between single-dose rifabutin and steady-state tipranavir/ritonavir 500 mg/200 mg (TPV/r) in healthy volunteers, abstr. A-456. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 26.Williams, R. L., M. L. Chen, and W. W. Hauck. 2002. Equivalence approaches. Clin. Pharmacol. Ther. 72:229-237. [DOI] [PubMed] [Google Scholar]