Abstract
The plasmid-mediated novel β-lactamase CTX-M-64 was first identified in Shigella sonnei strain UIH-1, which exhibited resistance to cefotaxime (MIC, 1,024 μg/ml) and ceftazidime (MIC, 32 μg/ml). The amino acid sequence of CTX-M-64 showed a chimeric structure of a CTX-M-15-like β-lactamase (N- and C-terminal moieties) and a CTX-M-14-like β-lactamase (central portion, amino acids 63 to 226), suggesting that it originated by homologous recombination between the corresponding genes. The introduction of a recombinant plasmid carrying blaCTX-M-64 conferred resistance to cefotaxime in Escherichia coli, and the activities of cefotaxime and ceftazidime were restored in the presence of clavulanic acid. Of note, CTX-M-64 production could also confer consistent resistance to ceftazidime, which differs from the majority of CTX-M-type enzymes, which poorly hydrolyze ceftazidime. These results were consistent with the kinetic parameters determined with the purified CTX-M-64 enzyme. The blaCTX-M-64 gene was flanked upstream by an ISEcp1 sequence and downstream by an orf477 sequence. The sequence of the 45-bp spacer region between the right inverted repeat (IRR) of ISEcp1 and blaCTX-M-64 was exactly identical to that of ISEcp1-blaCTX-M-15-like. Moreover, the presence of a putative IRR of ISEcp1 at the right end of truncated orf477 is indicative of an ISEcp1-mediated transposition event in the blaCTX-M-64 gene. The emergence of CTX-M-64 by probable homologous recombination would suggest the natural potential of an alternative mechanism for the diversification of CTX-M-type β-lactamases.
Shigellosis remains a public health concern throughout the world and has become an actual threat, particularly in developing countries, where 99% of the estimated 165 million annual episodes occur. Children under 5 years of age have been involved in more than half of the episodes and deaths (14). Shigellosis is more severe in malnourished children and elderly and immunocompromised people. Antibiotic treatment shortens the duration of clinical symptoms and pathogen excretion, prevents disease transmission, and reduces the risk of potential complications (18, 27, 34). However, empirical therapy with first-line antimicrobial agents, including ampicillin, trimethoprim-sulfamethoxazole, chloramphenicol, nalidixic acid, co-trimoxazole, and tetracycline, has become less effective due to the high prevalence of multidrug-resistant (MDR) clinical isolates among Shigella species (9, 28, 29, 32). For these MDR isolates, the therapeutic options for oral administration are fluoroquinolones for adults and oxyimino-cephalosporins for children.
Plasmid-encoded class A extended-spectrum β-lactamase (ESBL) production is still uncommon among Shigella species, despite the worldwide spread and prevalence of ESBL-producing clinical isolates belonging to the family Enterobacteriaceae. Four CTX-M-type β-lactamases, CTX-M-2, CTX-M-3, CTX-M-14, and CTX-M-15, and several TEM-derived ESBLs have been reported for Shigella sonnei (1, 11, 15, 25). S. sonnei strain UIH-1, characterized in this study, produced a novel CTX-M-type β-lactamase, a hybrid of the CTX-M-15-like β-lactamase, which is a new CTX-M-15 variant (GenBank accession no. DQ256091), and the CTX-M-14 β-lactamase; and this chimeric enzyme conferred resistance to ceftazidime as well as to cefotaxime and ceftriaxone.
MATERIALS AND METHODS
Clinical isolate.
S. sonnei UIH-1 was identified with the API 20E system (bioMérieux) in combination with tests for the utilization of citrate with Christensen's citrate medium (4), sodium acetate, and mucate and by PCR detection of the invE and ipaH genes with specific primer sets (Takara Bio, Shiga, Japan). Serological identification was performed with specific antisera (Denka Seiken, Tokyo, Japan).
Susceptibility testing.
β-Lactam MICs were measured by the microdilution broth method with a WalkAway-96 SI system (NEG Combo 6.11 J, NEG MIC 5 J, and ESBL plus panels; Dade Behring, Tokyo, Japan), according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (5, 21). Alternatively, for cefotaxime, ceftazidime, ceftriaxone, and aztreonam (Sigma), broth microdilution panels prepared in-house were used to provide a broader range of antimicrobial concentrations for evaluation of the MICs (5). Susceptibilities to non-β-lactams were tested by the disk diffusion method recommended by the CLSI (5). The susceptibility categories of the parent strain, the transformant, and the transconjugant were determined according to the criteria of the CLSI (6).
PCR detection and sequencing of β-lactamase gene.
Detection of the bla genes, including blaTEM, blaSHV, blaCTX-M-1, blaCTX-M-2, blaCTX-M-9, and blaCTX-M-8/25, was performed by PCR, as described previously (20). The additional primers used were consensus primers CTX-M/F′ and CTX-M/R1 (22) and primers CTX-M1A and CTX-M1B, which encompass the entire coding region (8). For sequence determination, the amplicons were purified with a QIAquick PCR purification kit (Qiagen), and both strands were directly sequenced with a BigDye Terminator cycle sequencing ready reaction kit and an ABI Prism model 3100 genetic analyzer (Applied Biosystems). The nucleotide and deduced amino acid sequences were analyzed with the BLAST program (http://www.ncbi.nlm.nih.gov/blast). The ClustalW program (http://www.ebi.ac.uk/clustalw) was used to align the amino acid sequences of multiple enzymes.
Plasmid conjugal transfer.
The conjugal transferability of the resistance determinants was investigated as described previously (20). Transconjugants were selected on bromothymol blue-lactose agar containing cefotaxime (20 μg/ml) and rifampin (rifampicin; 100 μg/ml; Sigma).
Cloning of blaCTX-M-64.
The conjugal plasmid was extracted and digested with EcoRI. The resultant fragments were ligated into the pCL1920 cloning vector (GenBank accession no. AB236930) and introduced into Escherichia coli XL-1 Blue. The transformants were selected on LB agar plates containing streptomycin (25 μg/ml; Sigma) and ampicillin (100 μg/ml; Sigma). The blaCTX-M-64 gene and its flanking region were amplified with the primers 5′-GGG GAT CCT TGC TCT GTG GAT AAC TTG CAG-3′ (the KpnI site is underlined) and 5′-CCC AAG CTT TCG GTG CAT AAA ACA CGG TG-3′ (the HindIII site is underlined). The product was digested with restriction enzymes and cloned into plasmid pCL1920. The resultant recombinant plasmid was introduced into E. coli XL-1 Blue, and transformants were selected as described above. To ensure that the enzyme was produced in the transformant, the nucleotide sequence of the insert was checked as described above.
Southern hybridization.
Plasmid DNA was prepared from bacterial cells by the alkaline extraction method (20). The DNAs were transferred to a positively charged nylon membrane (Clearblot N+ membrane; Atto Corp., Tokyo, Japan). The PCR product obtained with primers CTX-M/F′ and CTX-M/R1 (22) was labeled with digoxigenin-11-dUTP by use of a DIG High Prime DNA labeling and detection kit (Roche Applied Science). Hybridization and detection were performed according to the manufacturer's recommendations.
Purification of CTX-M-64 β-lactamase.
The blaCTX-M-64 gene was amplified with primers P1 (5′-GGA ATT CCA TAT GGT TAA AAA ATC ACT GCG-3′), which introduced an NdeI restriction site (underlined) to the 5′ end, and P2 (5′-CCC AAG CTT TTA CAA ACC GTC GGT GAC GAT-3′) which introduced an HindIII site (underlined) to the 3′ end. The amplified fragments were digested with the restriction enzymes and ligated into the pET29a vector (Novagen). Recombinant plasmid pET-CTX-M-64 was electroporated into E. coli BL21(DE3)pLysS after confirmation that the plasmid contained the blaCTX-M-64 gene sequence by sequencing analysis. The cells were cultured in 1 liter of LB broth supplemented with chloramphenicol (30 μg/ml; Sigma) and kanamycin (30 μg/ml; Sigma) at 37°C. Isopropyl-β-d-thiogalactopyranoside (final concentration, 0.5 mM) was added when the optical density of the culture at 600 nm reached 0.5, and the culture was incubated for an additional 3 h at 37°C. The cells were disrupted with a French press and centrifuged at 100,000 × g for 1 h. The supernatant containing the recombinant protein was loaded onto a HiTrap SP HP column (GE Healthcare) that had been preequilibrated with 50 mM morpholineethanesulfonic acid buffer (pH 6.0). The enzymes were eluted with a linear gradient of NaCl in the same buffer. The fractions with β-lactamase activity were loaded onto a Superdex 200 10/300GL column (GE Healthcare) and eluted with buffer (20 mM Tris-HCl, pH 7.5; 200 mM NaCl; 1 mM dithiothreitol). Finally, the eluted protein was concentrated and stored at −80°C until use. The purity of the β-lactamase was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie brilliant blue staining. The purified β-lactamase was also subjected to isoelectric focusing analysis with an Ampholine PAG plate (Amersham Biosciences).
Determination of kinetic parameters.
The kinetic parameters for the CTX-M-64 β-lactamase against various β-lactam substrates were measured at 37°C in 50 mM phosphate buffer (pH 7.0) with a spectrophotometer (Ultrospec 3000; Pharmacia Biotech). The values of the kinetic parameters Km and kcat were obtained from a Michaelis-Menten plot of the initial steady-state velocities (7, 30). Six different substrate concentrations were used to determine the parameters for each substrate. The absorption maxima of the substrates used were as follows: ampicillin, 235 nm; nitrocefin, 485 nm; cephalothin, 262 nm; ceftazidime, 274 nm; cefotaxime, 264 nm; and cefepime, 275 nm. The Km of poor substrates was determined as the competitive inhibition constant (Ki) from the competition assay between the substrate (ceftazidime) and nitrocefin (100 μM). The 50% inhibitory concentration was determined as the concentration of clavulanic acid that reduced the hydrolysis rate of 100 μM nitrocefin by 50% when the enzyme was preincubated with various concentrations of the inhibitor for 5 min at 37°C before addition of the substrate.
Nucleotide sequence accession number.
The nucleotide sequence data for blaCTX-M-64 of S. sonnei UIH-1 appear in the DDBJ/EMBL/GenBank database under accession no. AB284167.
RESULTS
Description of clinical isolate.
S. sonnei UIH-1 was recovered in August 2006 at the Urayasu Ichikawa City Hospital, Chiba, Japan, from a culture of a stool sample from a 37-year-old man with diarrhea, tenesmus, and fever that continued for 3 days after a trip to China. Serotyping revealed mostly smooth phase I colonies, but a small number of rough phase II variants were intermingled in the initial isolation culture. Both phase I and phase II strains of S. sonnei UIH-1 showed the same antibiograms with all antimicrobials tested. They were resistant to penicillins, cefotaxime (MIC, 1,024 μg/ml), ceftazidime (MIC, 32 μg/ml), ceftriaxone (1,024 μg/ml), cefpodoxime (MIC, >64 μg/ml), and aztreonam (32 μg/ml); and the activities of cefotaxime and ceftazidime were restored by clavulanic acid. The MICs of cephamycins, oxacephems, and carbapenems were within the susceptible range (Table 1). These isolates were also resistant to streptomycin, nalidixic acid, trimethoprim-sulfamethoxazole, and tetracycline.
TABLE 1.
MICs of β-lactams for S. sonnei clinical isolate UIH-1, the transconjugant, and the transformant
| Antibiotic | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| S. sonnei UIH-1 | E. coli χ1037 Rifr transconjugant | E. coli χ1037 Rifr | E. coli XL-1 Blue(pCL1920-CTX-M-64) | E. coli XL-1 Blue(pCL1920) | |
| Ampicillin | >16 | >16 | ≤2 | >16 | ≤2 |
| Amoxicillin-CLA | 2/1 | 2/1 | ≤1/0.5 | 8/4 | 2/1 |
| Piperacillin | >64 | >64 | ≤8 | >64 | ≤8 |
| Cefazolin | >16 | >16 | ≤1 | >16 | ≤1 |
| Cefotiam | >16 | >16 | ≤8 | >16 | ≤8 |
| Cefoperazone-SULa | 8/4 | ≤4/2 | ≤4/2 | >32/16 | ≤4/2 |
| Cefotaximec | 1,024 | 2,048 | ≤0.25 | >2,048 | ≤0.25 |
| Cefotaxime-CLAb | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 |
| Ceftazidimec | 32 | 64 | ≤0.25 | 2,048 | ≤0.25 |
| Ceftazidime-CLAb | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.5 | ≤0.12 |
| Ceftriaxonec | 1,024 | 2,048 | ≤0.25 | 2,048 | ≤0.25 |
| Cefpirome | >16 | >16 | ≤1 | >16 | ≤1 |
| Cefepime | >32 | >32 | ≤1 | >32 | ≤1 |
| Cefozopran | >16 | >16 | ≤1 | >16 | ≤1 |
| Cefaclor | >16 | >16 | ≤2 | >16 | ≤2 |
| Cefpodoxime | >64 | >64 | ≤0.5 | >64 | ≤0.5 |
| Cefoxitin | ≤2 | ≤2 | ≤2 | 8 | 4 |
| Cefmetazole | ≤0.5 | 1 | ≤0.5 | 1 | 1 |
| Cefotetan | ≤0.5 | ≤0.5 | ≤0.5 | 8 | ≤0.5 |
| Flomoxef | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Meropenem | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Aztreonamc | 32 | 64 | ≤0.25 | 1,024 | ≤0.25 |
SUL, sulbactam.
CLA, clavulanic acid at a fixed concentration of 4 μg/ml.
The antibiotic-containing plates were prepared in-house.
PCR and sequencing of bla gene.
A preliminary search for bla genes by the conventional PCR method failed to give positive results. However, PCR with blaCTX-M-specific consensus primers allowed the detection of a 520-bp fragment. Except for the primer sequences, the 478-bp nucleotide sequence contained a 450-bp sequence from its 5′ end that was identical to nucleotides 228 to 677 of the blaCTX-M-9 group and a 31-bp sequence from its 3′ end that was identical to nucleotides 675 to 705 of the blaCTX-M-1 group. On the basis of the finding that our blaCTX-M gene could have a blaCTX-M-9 group-blaCTX-M-1 group hybrid sequence, all possible combinations of primers from our stock were used in an attempt to amplify the structural gene by PCR, which resulted in the generation of an amplicon of the expected size with primers CTX-M1A and CTX-M1B. The sequence data for blaCTX-M indicated the presence of an open reading frame of 876 bp encoding a protein consisting of 291 amino acid residues. A BLAST search revealed 100% identity with blaCTX-M-15-like (GenBank accession no. DQ256091) from nucleotides 1 to 209 and nucleotides 675 to 876 and 100% identity with blaCTX-M-14 (GenBank accession no. AF252622) (or blaCTX-M-17, -21, -24, -27) from nucleotides 202 to 677. The deduced amino acid sequence showed 100% identity to the CTX-M-15-like β-lactamase derived from CTX-M-15 (GenBank accession no. AY044436) through a single Ala67-Pro substitution from amino acid residues 1 to 82 and amino acid residues 223 to 290 and 100% identity to the CTX-M-14 β-lactamase (or the CTX-M-9, -16, -17, -21, -24, and -27 β-lactamases) from amino acid residues 63 to 226 (Fig. 1). CTX-M-14 was expected to be the most probable variant forming the middle hybrid part because the highest prevalence of this enzyme among the CTX-M-9 group of enzymes described above has been noted in the Far East (3, 16, 19, 35). Thus, this novel CTX-M-type β-lactamase has been assigned the designation CTX-M-64 by G. A. Jacoby (http://www.lahey.org/studies/webt.asp), which differed from the CTX-M-15-like β-lactamase by 22 amino acid residues (92.4% similarity) and from the CTX-M-14 β-lactamase by 35 amino acid residues (88.0% similarity).
FIG. 1.
Comparison of the amino acid sequences of the CTX-M-64 β-lactamase with those of the CTX-M-15-like and CTX-M-14 β-lactamases. The complete sequence of CTX-M-64 is shown, and only differences in the sequence are indicated for the other two enzymes. Structural elements characteristic of class A β-lactamases are boxed. The amino acids of the omega loop are underlined.
Genetic environment of blaCTX-M-64.
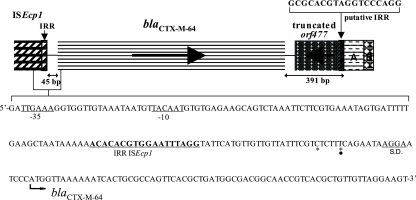
The flanking region of blaCTX-M-64 was determined (Fig. 2). The sequence of the spacer region between the right inverted repeat (IRR) of ISEcp1 and blaCTX-M-64 was exactly identical to that of ISEcp1-blaCTX-M-15-like (GenBank accession no. DQ256091), in which the length was 45 bp and in which there were 2 nucleotide substitutions from the corresponding ISEcp1-blaCTX-M-15 spacer region of 48 bp (GenBank accession no. AY044436). Cloning analysis revealed that a 345-bp 5′-truncated orf477 was located 47 bp downstream of the blaCTX-M-64 termination codon, in which the 5′ end of the orf477 was terminated by an 18-bp putative IRR of ISEcp1 (5′-GCGCACGTAGGTCCCAGG-3′) that was identical to a previously described IRR (26). The 112-bp sequence located immediately downstream of the truncated orf477 showed 100% identity with the sequence encoding the 3′ end of hypothetical protein 0115 and the start region of a hypothetical protein 0116 located on a large MDR plasmid of Salmonella enterica subsp. enterica serovar Newport (GenBank accession no. CP000604). The backbone plasmid of the MDR plasmid of 113,320 bp is shared by Yersinia pestis and has been detected in numerous MDR enterobacterial pathogens isolated from retail meat samples (31). The 112-bp sequence was followed by a 32-bp sequence which showed 90% identity with a 16-bp sequence encoding a 3′-truncated yadD homologue and a flanking spacer region of plasmid ColIb P-9 (GenBank accession no. AJ238399).
FIG. 2.
Schematic diagram of the blaCTX-M-64 gene and surrounding regions in S. sonnei UIH-1. For the upstream region of the blaCTX-M-64 gene, the nucleotide sequence of the 3′ end of an ISEcp1 element and the start region of bla are indicated. Putative −35 and −10 promoter sequences of the blaCTX-M-64 gene and the IRR sequence of ISEcp1 are underlined. A probable Shine-Dalgarno sequence (S.D.) is also underlined. Asterisks indicate the nucleotide substitutions from the corresponding ISEcp1-blaCTX-M-15 spacer sequence (GenBank accession no. AY044436). The closed circle indicates the nucleotide substitution from the corresponding chromosomal blaCTX-M-3 spacer sequence of Kluyvera ascorbata (GenBank accession no. AJ632119). The 391-bp sequence of the downstream region of the bla gene is 100% identical to the corresponding sequences of the K. ascorbata chromosomal blaCTX-M-3, including the putative IRR from ISEcp1. The sequence is followed by a downstream region showing sequence homology with hypothetical proteins located on a large MDR plasmid of Salmonella enterica subsp. enterica serovar Newport (GenBank accession no. CP000604) (block A), a 3′-truncated yadD homologue, and the flanking spacer region of plasmid ColIb P-9 (GenBank accession no. AJ238399) (block B).
Transfer of cefotaxime resistance and plasmid DNA analysis.
Cefotaxime resistance was transferred to E. coli at an approximate frequency of 1.1 × 10−5 CFU/donor cell. Electrophoretic analysis of the plasmid DNA revealed the transfer of a plasmid, and Southern blot hybridization analysis confirmed that the blaCTX-M-64 gene was located on this approximately 68-kb plasmid (data not shown).
Susceptibility testing.
The MICs of various β-lactams for the transconjugant and the transformant are listed in Table 1. Both the transconjugant and the transformant producing the CTX-M-64 enzyme conferred consistent resistance to cefotaxime, ceftriaxone, and aztreonam; on the other hand, they were susceptible to cephamycins and carbapenems. The reduction in the MIC of cefotaxime was observed by the addition of clavulanic acid. This trend is commonly observed in the majority of CTX-M-type β-lactamase producers. Of note, the CTX-M-64-producing transformant had a considerably augmented MIC of ceftazidime, which is thought to be a poor substrate for most CTX-M-type β-lactamases.
Purification and characterization of CTX-M-64 β-lactamase.
E. coli BL21(DE3)pLysS and the pET-29a vector were used for the overexpression of the blaCTX-M-64 gene for the purification of CTX-M-64. The optimized culture conditions yielded approximately 7 mg of purified CTX-M-64 enzyme per liter. The purified CTX-M-64 gave a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the pI of the enzyme was determined to be >8.7 (data not shown).
Kinetic parameters.
As shown in Table 2, CTX-M-64 showed high catalytic efficiencies (kcat/Km values) against ampicillin, nitrocefin, cephalothin, and cefotaxime, as is observed for other CTX-M-type β-lactamases. The 50% inhibitory concentration of clavulanic acid measured with nitrocefin as the substrate was 0.01 μM, and this result corroborated the inhibitor-sensitive nature of the CTX-M-64 enzyme. The catalytic activity (kcat) of CTX-M-64 against ceftazidime could not be determined due to its very high Ki value.
TABLE 2.
Kinetic parameter values for the CTX-M-64 β-lactamase
| Substrate | Km or Ki (μM) | kcat (s−1) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| Ampicillin | 19.5 ± 1.78 | 36.9 ± 0.89 | 1.9 × 106 |
| Nitrocefin | 14.8 ± 0.93 | 252 ± 5.10 | 1.7 × 107 |
| Cephalothin | 37.9 ± 1.06 | 185 ± 4.43 | 4.9 × 106 |
| Cefotaxime | 103 ± 13.6 | 197 ± 14.6 | 1.9 × 106 |
| Ceftazidimea | >104 | NDb | ND |
| Cefepime | 505 ± 68.7 | 67.7 ± 8.05 | 1.3 × 105 |
Nitrocefin (100 μM) was used as the reporter substrate to obtain the Kis value.
ND, not determined.
DISCUSSION
In this study, a novel chimeric β-lactamase, CTX-M-64, was first identified in a S. sonnei isolate recovered from a tourist who had returned from China. In Japan, approximately 80% of bacteriologically confirmed cases of shigellosis have been associated with tourists returning from foreign countries, especially from Asia, and S. sonnei has become the predominant cause of such enteric infections. Thus, it is important to monitor the emergence and the prevalence of the resistance mechanisms that may be introduced into the community by tourists who have traveled overseas and who are infected with enteropathogenic bacteria, including Shigella species.
In several survey studies, Woodford et al. (33) adopted multiplex PCR and Pitout et al. (23) adopted group-specific primers for the molecular classification of CTX-M-type β-lactamase genes. On the basis of the nucleotide sequence data of blaCTX-M-64, PCR with the primer sets used by Pitout et al. (23) are expected to fail to produce any amplification product. On the other hand, the use of a combination of CTX-M-1-group-specific forward primer and a CTX-M-9-group-specific reverse primer (2-bp mismatch) by Woodford et al. (33) may well generate a 205-bp PCR product from the blaCTX-M-64 gene, although a PCR product of this size would be indistinguishable from the PCR product generated from the blaCTX-M-9-group gene in their multiplex PCR system and would thus provide an incorrect result. Moreover, it appears to be certain that multiplex PCR methods are powerful tools for the classification of CTX-M-type β-lactamase genes, but hereafter, one will need to take into account the presence of hybrid-style β-lactamase genes like blaCTX-M-64 when the preexisting multiplex PCR fails to detect any of the known CTX-M-type β-lactamase genes.
CTX-M-64 showed high catalytic efficiencies against ampicillin, nitrocefin, cephalothin, and cefotaxime; and clavulanic acid behaved as a potent inhibitor of this enzyme. These enzymatic characteristics of CTX-M-64, as described above, are commonly observed in the majority of CTX-M-type enzymes. Additionally, CTX-M-64 had enhanced activity against ceftazidime, and this has been shown for a number of CTX-M-type enzymes. To evaluate further this property caused by CTX-M-64 production, we determined the kinetic parameters of CTX-M-64 against ceftazidime. Unfortunately, the catalytic efficiency (kcat/Km) of CTX-M-64 against ceftazidime could not be determined due to its very high Ki value. At present, two amino acid substitutions, Pro-167Ser and Asp-240Gly, have mainly been reported to be involved in the augmented hydrolytic activities of the CTX-M-type enzymes against ceftazidime (2, 12, 13, 24). Although the actual mechanism for the higher MIC of ceftazidime for CTX-M-64 producers remains uncertain, the glycine residue at position 240 in the CTX-M-64 enzyme probably plays a crucial role in the acquisition of the higher level of hydrolyzing activity against ceftazidime. In addition, it is speculated that the distinctive hybrid composition formed in CTX-M-64 might well cause particular steric interactions with ceftazidime and provide CTX-M-64 with its higher level of hydrolytic activity. Molecular modeling and X-ray crystallographic analyses would be needed to substantiate this speculation.
The blaCTX-M-64 gene was flanked upstream by an ISEcp1 sequence and downstream by an orf477 sequence. The presence of an ISEcp1 element upstream of the blaCTX-M-15 gene and an orf477 element downstream of the blaCTX-M-15 gene has been described previously (10), and ISEcp1 may contribute to the mobilization and high-level expression of the bla gene. Interestingly, the CTX-M-15-like enzyme has been identified in an E. coli clinical isolate in China, and the blaCTX-M-64 gene as well as the blaCTX-M-15-like gene has been located 45 bp downstream from ISEcp1, while the spacer region between ISEcp1 and blaCTX-M-15 is generally 48 bp in length (10, 17, 24). Moreover, the spacer sequences of the blaCTX-M-64 gene and the blaCTX-M-15-like gene shared two nucleotide substitutions from the corresponding sequence of the blaCTX-M-15 gene, whereas they shared only one of these two nucleotide substitutions from the corresponding chromosomal blaCTX-M-3 spacer sequence of Kluyvera ascorbata (GenBank accession no. AJ632119). The 391-bp region immediately downstream of the termination codon of the blaCTX-M-64 gene showed 100% sequence identity to the corresponding region of the K. ascorbata chromosomal blaCTX-M-3 (GenBank accession no. AJ632119), blaCTX-M-3 (GenBank accession no. AF550415), and blaCTX-M-15 (GenBank accession no. AY995206) genes. Moreover, the presence of a putative IRR of ISEcp1 described by Rodríguez et al. (26) at the right end of the 391-bp region is indicative of an ISEcp1-mediated transposition event. Thus, the blaCTX-M-15-like gene might have originated from the blaCTX-M-3 gene, which emerged by an independent mobilization event from the chromosome of a strain of K. ascorbata mediated by ISEcp1 inserted in its 45-bp upstream region (26). Then, the newly identified blaCTX-M-64 gene might have emerged by a double-crossover-type homologous recombination event between the blaCTX-M-15-like gene located on the approximately 68-kb plasmid and the blaCTX-M-14 gene possibly located on other plasmids coexisting in the same bacterial cell.
In conclusion, we report here on the emergence of the CTX-M-64 β-lactamase that shows a structure consisting of a chimera of two different CTX-M-type β-lactamase groups. In CTX-M-type β-lactamases, the acquisition of extended substrate specificity has so far been dependent on the accumulation of key amino acid substitutions that lead to changes in the steric interactions between the enzyme and the substrate agents (2, 12). Hereafter, however, it seems likely that the CTX-M-type β-lactamase would evolve to acquire the atypical substrate specificity through replacement of principal domains between cognate enzymes, as has been observed in CTX-M-64.
Acknowledgments
We are grateful to Kunikazu Yamane, Naohiro Shibata, and Kouji Kimura, Department of Bacterial Pathogenesis and Infection Control, National Institute of Infectious Diseases, for valuable scientific advice.
This work was supported by a grant (grant H18-Shinkou-011) from the Ministry of Health, Labor and Welfare of Japan.
We have no conflicts of interest.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Acikgoz, Z. C., Z. Gulay, M. Bicmen, S. Gocer, and S. Gamberzade. 2003. CTX-M-3 extended-spectrum β-lactamases in a Shigella sonnei clinical isolate: first report from Turkey. Scand. J. Infect. Dis. 35:503-505. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet, R., C. Recule, R. Baraduc, C. Chanal, D. Sirot, C. De Champs, and J. Sirot. 2003. Effect of D240G substitution in a novel ESBL CTX-M-27. J. Antimicrob. Chemother. 52:29-35. [DOI] [PubMed] [Google Scholar]
- 3.Chanawong, A., F. H. M'Zali, J. Heritage, J. H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen, W. B. 1949. Hydrogen sulfide production and citrate utilization in the differentiation of enteric pathogens and coliform bacteria. Research bulletin no. 1. Weld County Health Department, Greeley, CO.
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing. Eighteenth information supplement (M100-S18). Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Doi, Y., J. Wachino, M. Ishiguro, H. Kurokawa, K. Yamane, N. Shibata, K. Shibayama, K. Yokoyama, H. Kato, T. Yagi, and Y. Arakawa. 2004. Inhibitor-sensitive AmpC β-lactamase variant produced by an Escherichia coli clinical isolate resistant to oxyiminocephalosporins and cephamycins. Antimicrob. Agents Chemother. 48:2652-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutour, C., R. Bonnet, H. Marchandin, M. Boye, C. Chanal, D. Sirot, and J. Sirot. 2002. CTX-M-1, CTX-M-3, and CTX-M-14 β-lactamases from Enterobacteriaceae isolated in France. Antimicrob. Agents Chemother. 46:534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta, S., D. Dutta, P. Dutta, S. Matsushita, S. K. Bhattacharya, and S.-I. Yoshida. 2003. Shigella dysenteriae serotype 1: Kolkata, India. Emerg. Infect. Dis. 9:1471-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert, C., V. Gautier, and G. Arlet. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 57:14-23. [DOI] [PubMed] [Google Scholar]
- 11.Kim, S., J. Kim, Y. Kang, Y. Park, and B. Lee. 2004. Occurrence of extended-spectrum β-lactamases in members of the genus Shigella in the Republic of Korea. J. Clin. Microbiol. 42:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, S., M. Ishiguro, Y. Ishii, J. Alba, and K. Yamaguchi. 2004. Role of a mutation at position 167 of CTX-M-19 in ceftazidime hydrolysis. Antimicrob. Agents Chemother. 48:1454-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura, S., Y. Ishii, K. Tateda, and K. Yamaguchi. 2007. Predictive analysis of ceftazidime hydrolysis in CTX-M-type β-lactamase family members with a mutational substitution at position 167. Int. J. Antimicrob. Agents 29:326-331. [DOI] [PubMed] [Google Scholar]
- 14.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation. Bull. W. H. O. 77:651-656. [PMC free article] [PubMed] [Google Scholar]
- 15.Lartigue, M. F., L. Poirel, J. W. Decousser, and P. Nordmann. 2005. Multidrug-resistant Shigella sonnei and Salmonella enterica serotype Typhimurium isolates producing CTX-M β-lactamases as causes of community-acquired infection in France. Clin. Infect. Dis. 40:1069-1070. [DOI] [PubMed] [Google Scholar]
- 16.Li, H., and J. B. Li. 2005. Detection of five novel CTX-M-type extended spectrum beta-lactamases with one to three CTX-M-14 point mutations in isolates from Hefei, Anhui Province, China. J. Clin. Microbiol. 43:4301-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2007. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 18.Mahoney, F. J., T. A. Farley, D. F. Burbank, N. H. Leslie, and L. M. McFarland. 1993. Evaluation of an intervention program for the control of an outbreak of shigellosis among institutionalized persons. J. Infect. Dis. 168:1177-1180. [DOI] [PubMed] [Google Scholar]
- 19.Munday, C. J., J. Xiong, C. Li, D. Shen, and P. M. Hawkey. 2004. Dissemination of CTX-M type β-lactamases in Enterobacteriaceae isolates in the People's Republic of China. Int. J. Antimicrob. Agents 23:175-180. [DOI] [PubMed] [Google Scholar]
- 20.Nagano, N., C. Cordevant, and Y. Nagano. 2008. Upper and lower urinary tract infection caused by Klebsiella pneumoniae serotype K2 and CTX-M-15 β-lactamase-producing serotype K1: a case report and characterization of serum killing resistance. J. Med. Microbiol. 57:121-124. [DOI] [PubMed] [Google Scholar]
- 21.Nagano, N., Y. Nagano, C. Cordevant, N. Shibata, and Y. Arakawa. 2004. Nosocomial transmission of CTX-M-2 β-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J. Clin. Microbiol. 42:3978-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pimkin, M., and M. Edelstein. 2005. Improved PCR detection and subtyping of CTX-M-β-lactamase-encoding genes, abstr. 649. Abstr. 15th Eur. Congr. Clin. Microbiol. Infect. Dis.
- 23.Pitout, J. D. D., A. Hossain, and N. D. Hanson. 2004. Phenotypic and molecular detection of CTX-M-β-lactamases produced by Escherichia coli and Klebsiella spp. J. Clin. Microbiol. 42:5715-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum ß-lactamase CTX-M-15 and of its structurally related ß-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 25.Radice, M., C. González, P. Power, M. C. Vidal, and G. Gutkind. 2001. Third-generation cephalosporin resistance in Shigella sonnei, Argentina. Emerg. Infect. Dis. 7:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez, M. M., P. Power, M. Radice, C. Vay, A. Famiglietti, M. Galleni, J. A. Ayala, and G. Gutkind. 2004. Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: a possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrob. Agents Chemother. 48:4895-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salam, M. A., and M. L. Bennish. 1991. Antimicrobial therapy for shigellosis. Rev. Infect. Dis. 13(Suppl. 4):S332-S341. [DOI] [PubMed] [Google Scholar]
- 28.Sivapalasingam, S., J. M. Nelson, K. Joyce, M. Hoekstra, F. J. Angulo, and E. D. Mintz. 2006. High prevalence of antimicrobial resistance among Shigella isolates in the United States tested by the National Antimicrobial Resistance Monitoring System from 1999 to 2002. Antimicrob. Agents Chemother. 50:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Seidlein, L., D. R. Kim, M. Ali, H. Lee, X. Wang, V. D. Thiem, G. Canh do, W. Chaicumpa, M. D. Aqtini, A. Hossain, Z. A. Bhutta, C. Mason, O. Sethabutr, K. Talukder, G. B. Nair, J. L. Deen, K. Kotloff, and J. Clemens. 2006. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 3:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wachino, J., H. Kurokawa, S. Suzuki, K. Yamane, N. Shibata, K. Kimura, Y. Ike, and Y. Arakawa. 2006. Horizontal transfer of blaCMY-bearing plasmids among clinical Escherichia coli and Klebsiella pneumoniae isolates and emergence of cefepime-hydrolyzing CMY-19. Antimicrob. Agents Chemother. 50:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch, T. J., W. F. Fricke, P. F. McDermott, D. G. White, M. L. Rosso, D. A. Rasko, M. K. Mammel, M. Eppinger, M. J. Rosovitz, D. Wagner, L. Rahalison, J. E. Leclerc, J. M. Hinshaw, L. E. Lindler, T. A. Cebula, E. Carniel, and J. Ravel. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson, G., J. M. Easow, C. Mukhopadhyay, and P. G. Shivananda. 2006. Isolation & antimicrobial susceptibility of Shigella from patients with acute gastroenteritis in western Nepal. Indian J. Med. Res. 123:145-150. [PubMed] [Google Scholar]
- 33.Woodford, N., E. J. Fagan, and M. J. Ellington. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum ß-lactamases. J. Antimicrob. Chemother. 57:154-155. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2005. Shigellosis: disease burden, epidemiology and case management. Wkly. Epidemiol. Rec. 80:94-99. [PubMed] [Google Scholar]
- 35.Yu, W. L., P. L. Winokur, D. L. Von Stein, M. A. Pfaller, J. H. Wang, and R. N. Jones. 2002. First description of Klebsiella pneumoniae harboring CTX-M beta-lactamases (CTX-M-14 and CTX-M-3) in Taiwan. Antimicrob. Agents Chemother. 46:1098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]