Abstract
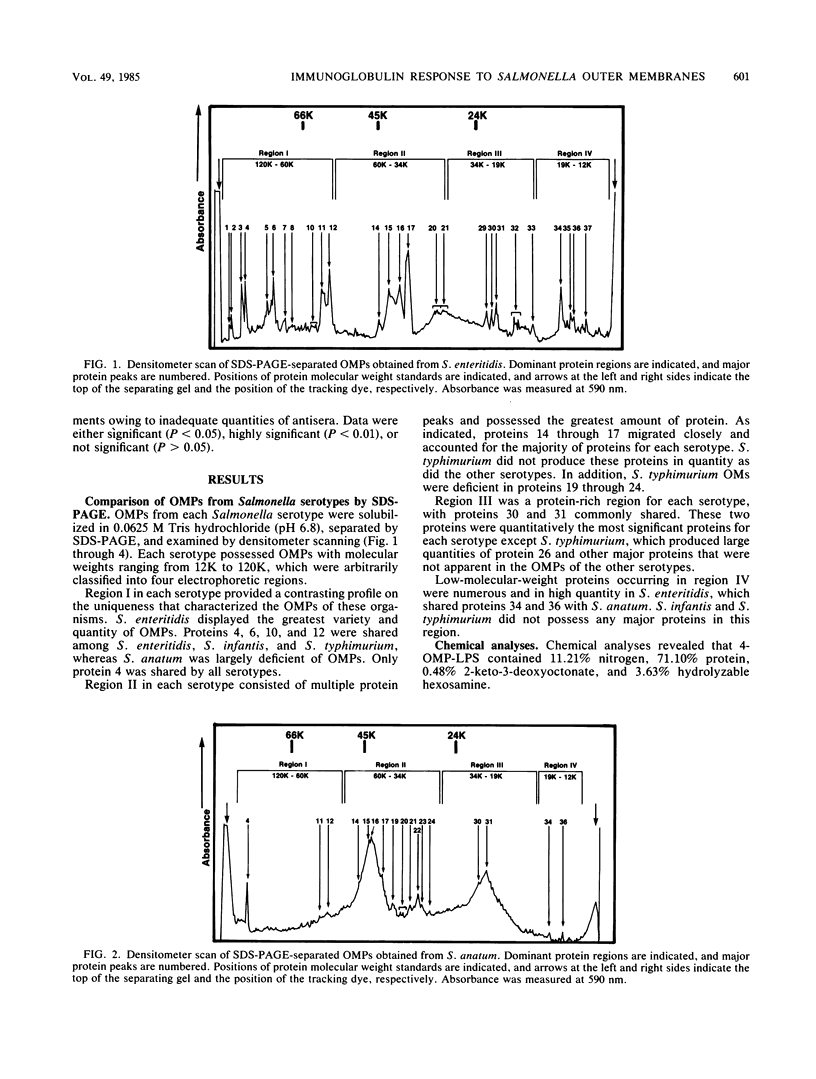
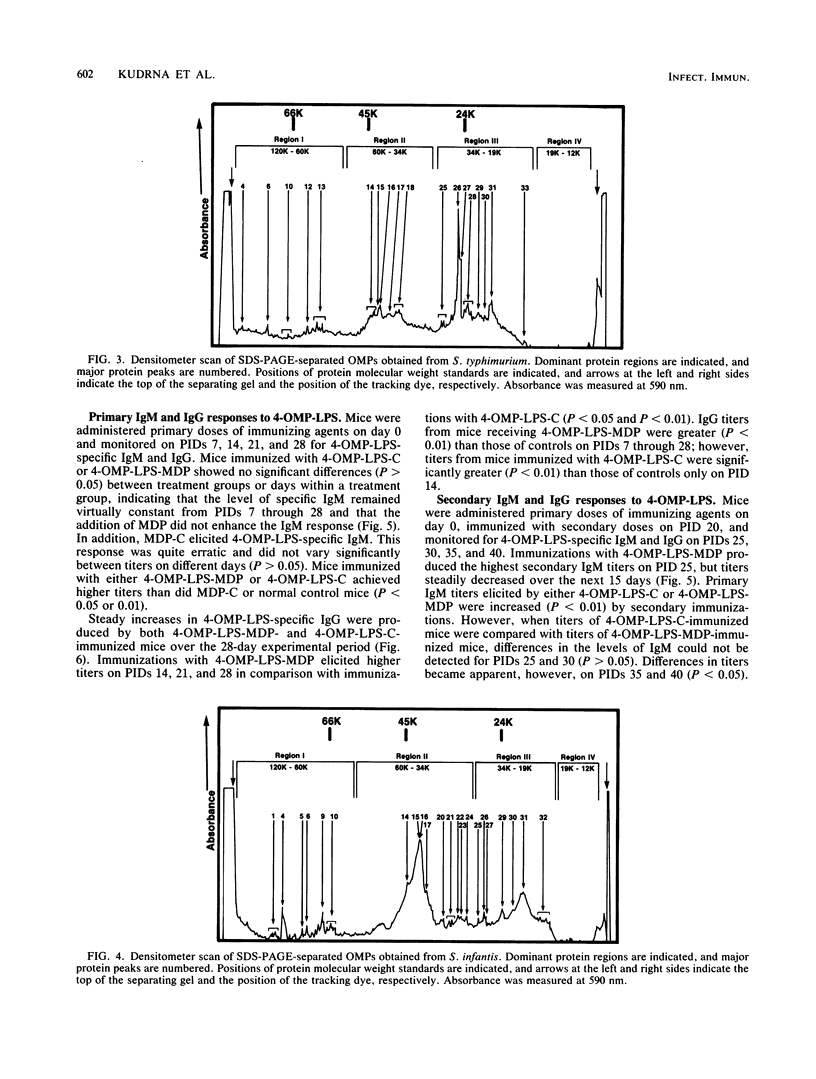
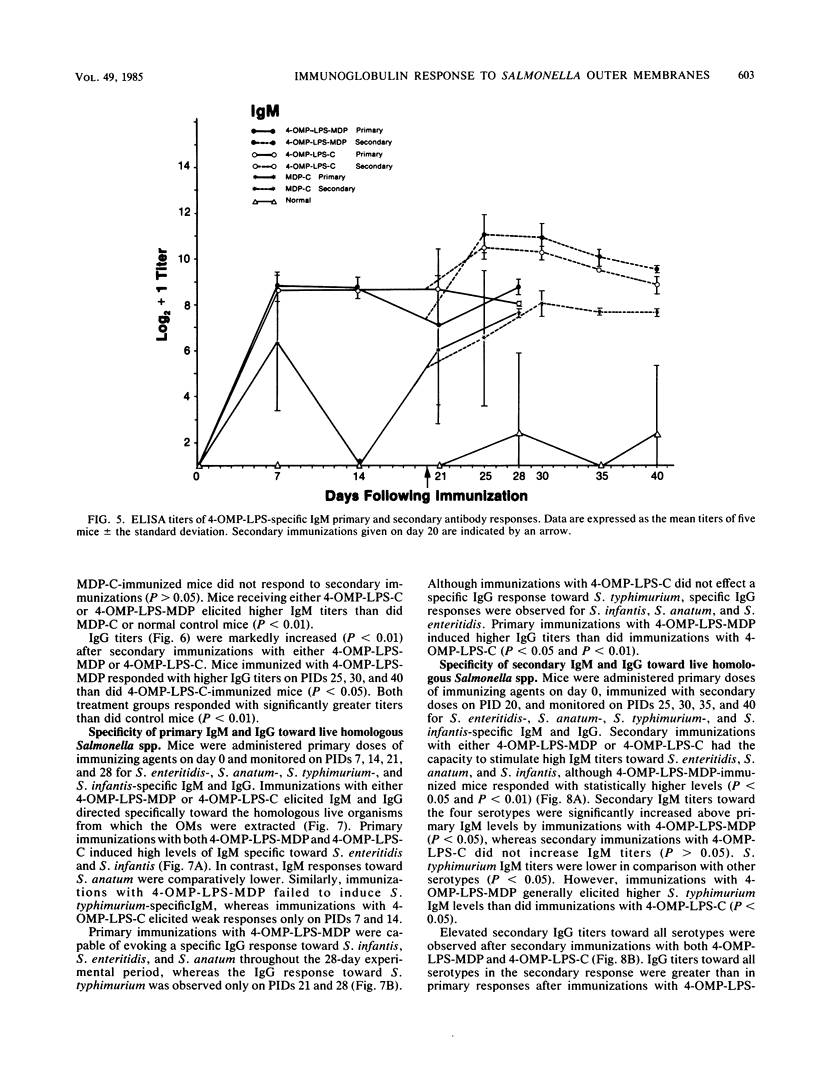
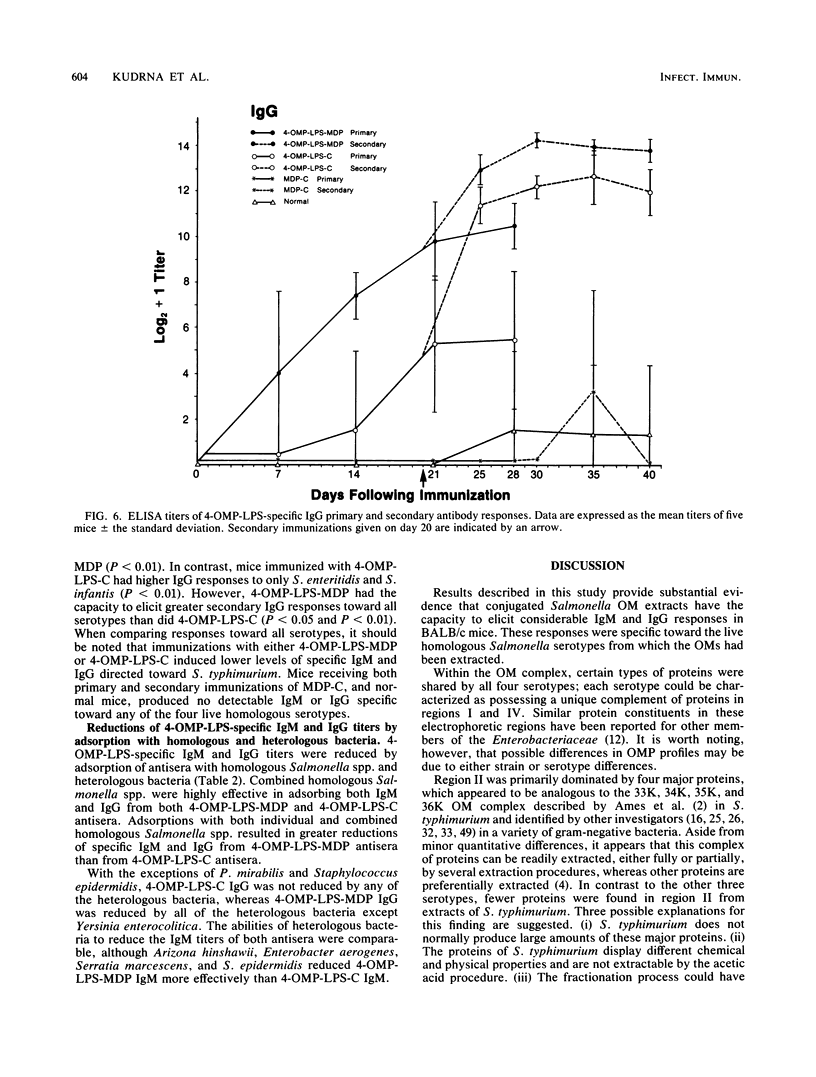
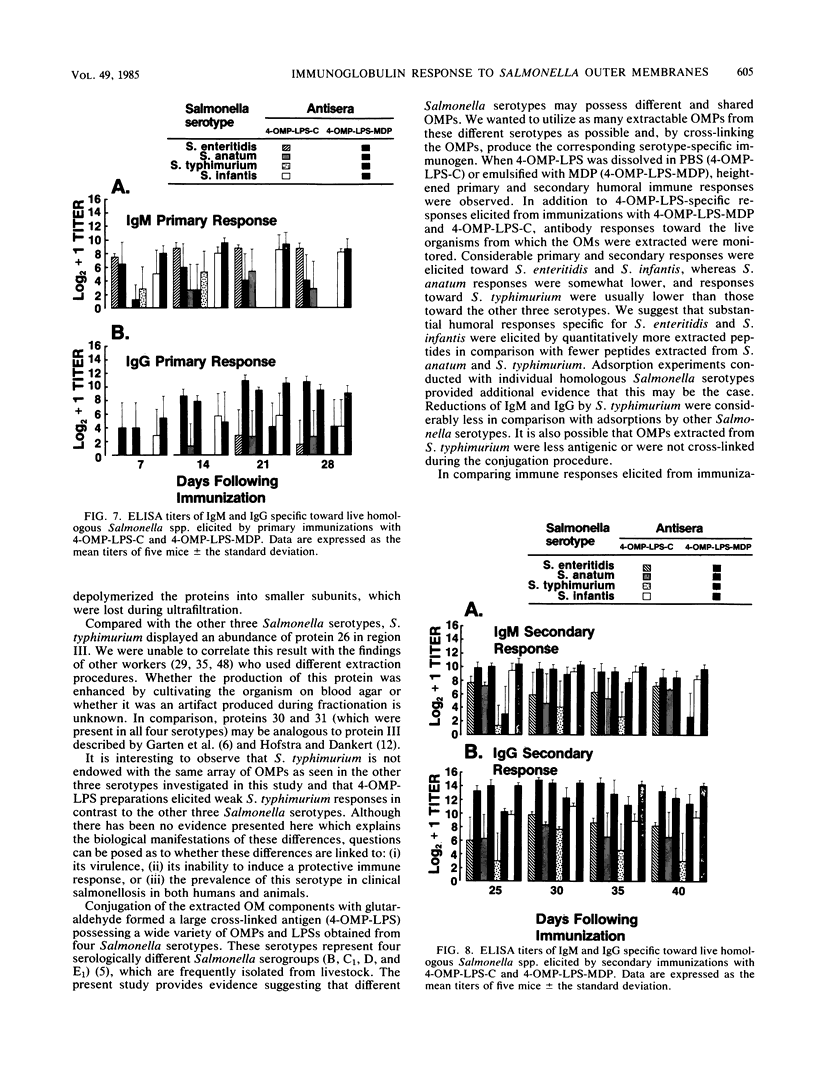
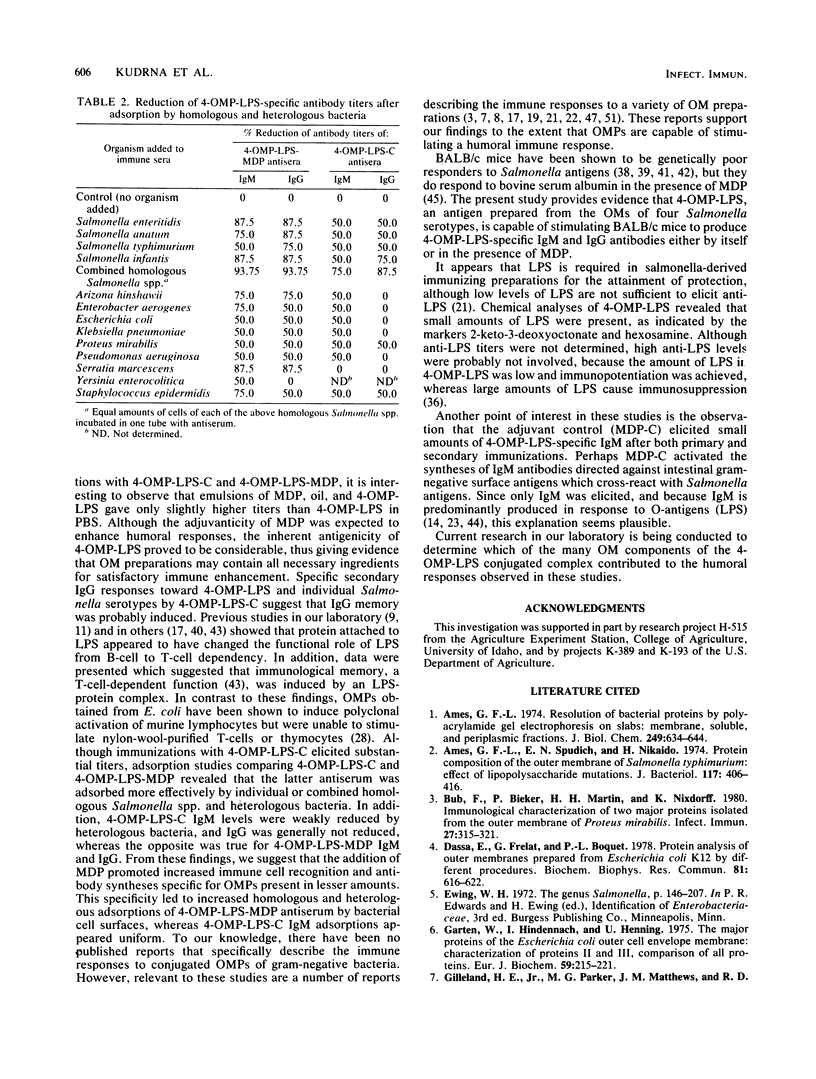
Outer membranes (OMs) of Salmonella enteritidis, S. anatum, S. typhimurium, and S. infantis were extracted and cross-linked with glutaraldehyde to form a large macromolecular antigen. The antigen consisted of OM proteins and lipopolysaccharide and was designated 4-OMP-LPS. Polyacrylamide gel electrophoresis of extracted OMs from each serotype revealed differences in protein profiles. S. enteritidis and S. infantis possessed a greater variety of proteins than did S. anatum and S. typhimurium. Immunizations with 4-OMP-LPS in phosphate-buffered saline (4-OMP-LPS-C) and 4-OMP-LPS emulsified with muramyl dipeptide in the oil phase of a hexadecane-water emulsion (4-OMP-LPS-MDP) revealed that BALB/c mice were capable of eliciting specific primary and secondary immunoglobulin M (IgM) and IgG responses. Both antigen preparations were capable of eliciting IgM and IgG specific for the cell surfaces of each live Salmonella serotype. Also, 4-OMP-LPS-MDP and 4-OMP-LPS-C were capable of evoking a substantial anamnestic response. Adsorption studies revealed that the combined serotypes had the antigenic capacity to adsorb up to 94% of the antibodies, but 4-OMP-LPS-MDP antibodies were more effectively adsorbed than were 4-OMP-LPS-C antibodies. Adsorption of pooled antiserum with heterologous bacteria yielded a variety of adsorption profiles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub F., Bieker P., Martin H. H., Nixdorff K. Immunological characterization of two major proteins isolated from the outer membrane of Proteus mirabilis. Infect Immun. 1980 Feb;27(2):315–321. doi: 10.1128/iai.27.2.315-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E., Frelat G., Boquet P. L. Protein analysis of outer membranes prepared from Escherichia coli K 12 by different procedures. Biochem Biophys Res Commun. 1978 Mar 30;81(2):616–622. doi: 10.1016/0006-291x(78)91580-2. [DOI] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Parker M. G., Matthews J. M., Berg R. D. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984 Apr;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R. C., Pavlovskis O. R., Galloway D. R. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984 Jan;43(1):49–53. doi: 10.1128/iai.43.1.49-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepper K. P., Garman R. D., Lyons M. F., Teresa G. W. Plaque-forming cell response in BALB/c mice to two preparations of LPS extracted from Salmonella enteritidis. J Immunol. 1979 Apr;122(4):1290–1293. [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. F., Regan K. M., Foss C. L., Teresa G. W. Induction of immunologic memory by a lipopolysaccharide-protein complex isolated from Fusobacterium necrophorum: cellular response. Am J Vet Res. 1982 Jan;43(1):117–121. [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Antigenic cross-reactivity of major outer membrane proteins in enterobacteriaceae species. J Gen Microbiol. 1979 Apr;111(2):293–302. doi: 10.1099/00221287-111-2-293. [DOI] [PubMed] [Google Scholar]
- Ison C. A., Hadfield S. G., Glynn A. A. Enzyme-linked immunosorbent assay (ELISA) to detect antibodies in gonorrhea using whole cells. J Clin Pathol. 1981 Sep;34(9):1040–1043. doi: 10.1136/jcp.34.9.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. M. Immunomodulatory effects of bacterial lipopolysaccharide. J Immunopharmacol. 1981;3(2):119–132. doi: 10.3109/08923978109026423. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Karch H., Gmeiner J., Nixdorff K. Alteration of the immunoglobulin G subclass responses in mice to lipopolysaccharide: effects of nonbacterial proteins and bacterial membrane phospholipids or outer membrane proteins of Proteus mirabilis. Infect Immun. 1983 Apr;40(1):157–165. doi: 10.1128/iai.40.1.157-165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Modulation of the IgG subclass responses to lipopolysaccharide by bacterial membrane components: differential adjuvant effects produced by primary and secondary stimulation. J Immunol. 1983 Jul;131(1):6–8. [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxén H., Mäkelä P. H. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect Immun. 1981 Nov;34(2):328–332. doi: 10.1128/iai.34.2.328-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landy M., Sanderson R. P., Jackson A. L. Humoral and cellular aspects of the immune response to the somatic antigen of Salmonella enteritidis. J Exp Med. 1965 Sep 1;122(3):483–504. doi: 10.1084/jem.122.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Mohri S., Watanabe T., Nariuchi H. Studies of the immunological activities of the outer membrane protein from Escherichia coli. Immunology. 1982 Jun;46(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Odumeru J. A., Ronald A. R., Albritton W. L. Characterization of cell proteins of Haemophilus ducreyi by polyacrylamide gel electrophoresis. J Infect Dis. 1983 Oct;148(4):710–714. doi: 10.1093/infdis/148.4.710. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Protein neighborhoods in the outer membrane of Salmonella typhimurium. Biochim Biophys Acta. 1980 Feb 28;596(2):235–247. doi: 10.1016/0005-2736(80)90358-2. [DOI] [PubMed] [Google Scholar]
- Persson U. Lipopolysaccharide-induced suppression of the primary immune response to a thymus-dependent antigen. J Immunol. 1977 Mar;118(3):789–796. [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Plant J. E., Wilson B. M., Glynn A. A. The protein-lipopolysaccharide complex extracted with trichloracetic acid from Salmonella typhimurium effective in protection of mice against S. typhimurium infection. Parasite Immunol. 1982 Jul;4(4):259–271. doi: 10.1111/j.1365-3024.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Scibienski R. J. Cellular parameters of the immunological memory induced by lysozyme-LPS mixtures and complexes. Immunol Commun. 1979;8(3):325–336. doi: 10.3109/08820137909050046. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Staruch M. J., Wood D. D. Genetic influences on the adjuvanticity of muramyl dipeptide in vivo. J Immunol. 1982 Jan;128(1):155–160. [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard L. F., Renshaw H. W., Burger D. Cell-mediated immunity in neonatal calves: delayed-type hypersensitivity and lymphocyte blastogenesis following immunization with a mycobacterial immunopotentiating glycolipid and tuberculoproteins of Mycobacterium bovis. Am J Vet Res. 1978 Apr;39(4):579–584. [PubMed] [Google Scholar]


