Abstract
An international interlaboratory proficiency testing program for the measurement of antifungal drugs was initiated in 2007. This first round was limited to azole antifungals: fluconazole, itraconazole and hydroxyitraconazole, voriconazole, and posaconazole. The results demonstrate the need for and utility of an ongoing proficiency testing program to further improve the analytical methods for routine patient management and clinical research.
The measurement of plasma concentrations of azole antifungals is often used in a research setting to assess the pharmacokinetic behavior of these drugs and to characterize their pharmacokinetic drug-drug interactions. In the clinical setting, reports have emerged on the relationship between plasma concentrations and efficacy or toxicity for selected azoles, suggesting the usefulness of therapeutic drug monitoring (TDM) of azole antifungals, especially for itraconazole (ITZ) (3), voriconazole (VRZ) (6), and posaconazole (PSZ) (7). By means of TDM, a clinician is able to individualize a drug dosage to improve its efficacy and reduce toxicity by optimizing target attainment. The TDM of azoles has now been included in the updated guidelines for the treatment of invasive aspergillosis by the Infectious Diseases Society of America (IDSA) (9).
The wide application of analytical methods for azole antifungal drugs requires intralaboratory and interlaboratory quality control (QC) procedures to ensure that these methods have sufficient accuracy, precision, and specificity. Participation in interlaboratory proficiency testing programs is common practice for many infectious disease drugs (1, 4, 5, 8), but such a program incorporating all currently marketed azole antifungal drugs has not been available so far. Therefore, we initiated an international interlaboratory proficiency testing program for the measurement of azole antifungal agents.
Methods.
Drug-free plasma from selected healthy volunteers was obtained through plasmapheresis with 100 ml of 4% sodium citrate and was provided by the Dutch Blood Bank (Sanquin, Nijmegen, The Netherlands). All antifungal azoles were obtained from pharmaceutical companies and had a very high (>99%) specified purity. Purity was checked based on analytical certificates provided by the supplier of the drug. Samples were prepared using 3 ml of human plasma that was spiked with fluconazole (FLZ), ITZ, hydroxyitraconazole (hITZ), VRZ, and PSZ. The azole compounds were weighed out on an independently calibrated balance, subsequently dissolved in methanol, and diluted with blank human plasma to obtain five different samples. Sample 1 contained a combination of FLZ, ITZ, hITZ, and VRZ (Table 1). Samples 2 and 3 contained a low and high concentration, respectively, of VRZ. Samples 4 and 5 contained a low and high concentration, respectively, of PSZ. The QC samples were dispensed in polypropylene vials and stored at −40°C until shipment. Stability at −40°C, 4°C, and ambient temperature (including the daylight environment at ambient temperature) was proven for at least 14 days, including three freeze-thaw cycles. All samples were dispatched at ambient temperature.
TABLE 1.
Concentrations (in mg/liter) of the azole antifungals in the QC samples
| Sample | FLZ | ITZ | hITZ | VRZ | PSZ |
|---|---|---|---|---|---|
| 1 | 8.17 | 0.55 | 1.01 | 2.11 | |
| 2 | 0.51 | ||||
| 3 | 2.03 | ||||
| 4 | 3.00 | ||||
| 5 | 0.30 |
All weighed-in concentrations were considered true values, and the obtained concentrations were all within a concentration range that is generally achieved after the oral or intravenous administration of azole drugs. The samples were analyzed with our own validated high-performance liquid chromatography (HPLC) method as a confirmative check (less than a 5% deviation from the true concentration) before the samples were released for the QC program.
Forty laboratories from four different continents (North America, Europe, Asia, and Australia) whose scientists have published on the bioanalysis of antifungal azoles were invited to participate, free of charge, in the first round of the QC program. The participants were requested to analyze the samples within 6 weeks after they were dispatched and were asked to provide details about their analytical methods.
Descriptive statistics were calculated after the standardization of all laboratory results to percentages with reference to the true value. The deviation from the true concentration (inaccuracy) was calculated by the subtraction of 100% from these percentages. Concentrations within 20% of the weighed-in concentration were considered to be satisfactory or correct (2). A one-way analysis of variance was performed to determine whether the drug to be analyzed was of influence on the absolute inaccuracies of the laboratories. An unpaired t test was performed on the absolute inaccuracies to determine a difference in the analytical methods. A paired samples t test was performed on the absolute inaccuracies, in order to determine if there was a difference between performances for the high versus low concentrations of PSZ and VRZ. All statistical analyses were performed using SPSS 14.0 (SPSS, Inc., Chicago, IL). A P value of <0.05 was considered statistically significant. All participants were provided feedback on their performance within 3 months after the first rounds' deadline. The results from the other laboratories were reported anonymously.
Results.
Thirty-six laboratories subscribed to the program; 33 laboratories returned results that could be evaluated. The laboratories used HPLC with either fluorescence, UV, or diode-array detection (n = 26), liquid chromatography-mass spectrometry (LC-MS; n = 6), or bioassay (n = 3) techniques to measure the azoles. Two laboratories used a combination of techniques to measure different azole antifungal drugs. None of the laboratories reported lower limits of quantitation that were above the spiked concentrations, indicating the suitability of these methods in clinical practice.
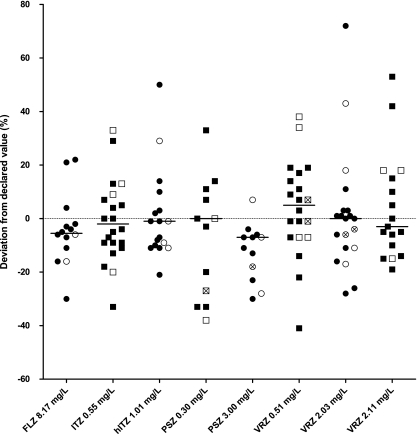
The percentages of correct analyses within the predefined range of 80 to 120% of the weighed-in concentrations were as follows: FLZ, 79% (n = 14 analyses); ITZ, 78% (n = 23); hITZ, 78% (n = 18); VRZ, 82% (n = 57); and PSZ, 62% (n = 26) (Fig. 1). The mean absolute inaccuracy (and 95% confidence interval) for each specific method was 20.74 (12.36 to 29.11) for HPLC, 17.70 (13.13 to 22.28) for LC-MS, and 6.33 (−0.59 to 13.25) for bioassays. No difference in the absolute inaccuracies was observed in the performance of HPLC with UV or fluorescence detection versus LC-MS techniques (P = 0.615, unpaired t test). No comparison could be made with regard to the bioassays since the numbers were too small to make a valid statement. All of the participating laboratories using a bioassay reported adequate values within the 80 to 120% range. Laboratories using the bioassay were not able to measure the concentrations of azole antifungals in the combined sample. A one-way analysis of variance yielded no statistically significant differences between the absolute inaccuracies related to the different azole antifungal drugs [F(4,86) = 0.884; P = 0.477]. All results were correctly reported by 18 of 33 laboratories (54.5%).
FIG. 1.
Deviation from the declared value. The horizontal solid lines represent the median value. The filled symbols indicate HPLC results, the unfilled symbols indicate LC-MS results, and the unfilled symbols with crosses indicate bioassay results. Four data points are outside the axis limits and are not shown in the graph: one laboratory reported ITZ at 17,595%, another laboratory reported hITZ at 126%, and a third laboratory reported 0.30 mg/liter posaconazole at 300% and 3.00 mg/liter posaconazole at 231%.
The performance for the lowest concentration of PSZ was less accurate than for the higher concentration (54% versus 69% correct analyses), but the test did not yield a statistical significance (n = 13; P = 0.130, paired t test). For VRZ, there was no difference between the high and low concentrations. Laboratories were informed about their performance to enable them to improve their methods.
Discussion.
The purpose of our proficiency testing program for the TDM of azole antifungals is to alert laboratories of potential problems and is therefore a useful instrument to monitor the accuracy of analytical methods.
The first results of this new QC program of azole antifungal agents show a performance ranging between 62 and 82% and thus clearly indicate the need to further improve analytical methods. The data suggest that in particular, analysis of PSZ, although not statistically significant from the other azoles, requires attention. Inaccurate results may introduce bias in pharmacokinetic studies or may provide a basis for incorrect dose adjustments in TDM.
For a first round, the number of participating labs is relatively high. However, even with this great contribution from all participants, there are some minor drawbacks. Very low and high concentrations outside the therapeutic range have not yet been included so we were not able to elude problems with lower limits of quantitation. Dispatching a combined sample of azoles may provide efficiency in determining difficulties with specificity and/or selectivity; however, this precludes laboratories using bioassays from participation. Sources of error in the current program have not been established. Therefore, no conclusions from this round on the reasons of inadequate performance can be drawn. Future rounds will address potential explanations for these shortcomings in more detail. These future rounds are needed to establish whether the program indeed contributes to the improvement of antifungal azole analysis. The program will also be extended to more laboratories and more antifungal drugs.
Acknowledgments
We express gratitude to our analytical staff (Corrien Verweij-van Wissen) and the office of the KKGT (Anneke Harteveld and Jaco Eerland) for the preparation of the QC samples.
We are indebted to (in alphabetical order) Janssen Pharmaceuticals, Pfizer, Inc., and Schering-Plough for the supply of FLZ (Pfizer), ITZ and hITZ (Janssen Pharmaceuticals), VRZ (Pfizer), and PSZ (Schering-Plough).
All authors have contributed to setting up the program and have read and improved the manuscript for publication.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Aarnoutse, R. E., C. P. Verweij-van Wissen, E. W. van Ewijk-Beneken Kolmer, E. W. Wuis, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2002. International interlaboratory quality control program for measurement of antiretroviral drugs in plasma. Antimicrob. Agents Chemother. 46:884-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2001. Guidance for industry. Bioanalytical method validation. Center for Drug Evaluation and Research and Center for Veterinary Medicine, Food and Drug Administration, U.S. Department of Health and Human Services, Washington, DC. http://www.fda.gov/cder/guidance/4252fnl.htm.
- 3.Dominguez-Gil, H. A., N. A. Sanchez, and M. J. Garcia Sanchez. 2006. Therapeutic drug monitoring of itraconazole and the relevance of pharmacokinetic interactions. Clin. Microbiol. Infect. 12(Suppl. 7):97-106.16460557 [Google Scholar]
- 4.Droste, J. A., R. E. Aarnoutse, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2003. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J. Acquir. Immune Defic. Syndr. 32:287-291. [DOI] [PubMed] [Google Scholar]
- 5.Holland, D. T., R. DiFrancesco, J. Stone, F. Hamzeh, J. D. Connor, and G. D. Morse. 2004. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob. Agents Chemother. 48:824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 7.Smith, J., and D. Andes. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 30:167-172. [DOI] [PubMed] [Google Scholar]
- 8.Steele, B. W., E. Wang, G. E. Palomaki, G. G. Klee, R. J. Elin, S. J. Soldin, and D. L. Witte. 2001. An evaluation of analytic goals for assays of drugs: a College of American Pathologists Therapeutic Drug Monitoring Survey Study. Arch. Pathol. Lab. Med. 125:729-735. [DOI] [PubMed] [Google Scholar]
- 9.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]