Abstract
Yessotoxin (YTX) is a globally distributed marine toxin produced by some isolates of the dinoflagellate species Protoceratium reticulatum, Lingulodinium polyedrum, and Gonyaulax spinifera within the order Gonyaulacales. The process of isolating cells and testing each isolate individually for YTX production during toxic blooms are labor intensive, and this impedes our ability to respond quickly to toxic blooms. In this study, we used molecular sequences from the large subunit and internal transcribed spacer genomic regions in the ribosomal operon of known YTX-producing dinoflagellates to determine if genetic differences exist among geographically distinct populations or between toxic and nontoxic isolates within species. In all analyses, all three YTX-producing species fell within the Gonyaulacales order in agreement with morphological taxonomy. Phylogenetic analyses of available rRNA gene sequences indicate that the capacity for YTX production appears to be confined to the order Gonyaulacales. These findings indicate that Gonyaulacoloid dinoflagellate species are the most likely to produce YTX and thus should be prioritized for YTX screening during events. Dinoflagellate species that fall outside of the Gonyaulacales order are unlikely to produce YTX. Although the rRNA operon offers multiple sequence domains to resolve species level diversification within this dinoflagellate order, these domains are not sufficiently variable to provide robust markers for YTX toxicity.
Yessotoxin (YTX) is a globally distributed toxin originally isolated from scallops, Patinopecten yessoensis, for which the toxin class was named (36). Due to the potential public health implications of ingesting contaminated shellfish, YTX is a regularly monitored marine toxin in New Zealand, Europe, and Japan. In 2002, the European Commission placed YTX into a separate phycotoxin group and established a regulatory level of 1 μg g−1 of YTX equivalents in shellfish meat intended for human consumption (directive 2002/225/EC); Japan and New Zealand have similar regulatory language.
There are three confirmed producers of the YTX phycotoxin: the dinoflagellates Protoceratium reticulatum (Claparhde and Lachmann) Buetschli, Lingulodinium polyedrum (Stein) Dodge, and Gonyaulax spinifera Dodge. YTX production within and among dinoflagellate species tested to date is highly variable. Previous reports have identified both nontoxic and toxic isolates of L. polyedrum from Spain, the United Kingdom, and California (3, 18, 43, 47, 58). In addition, several YTX-positive isolates of L. polyedrum have been identified in Italy (9, 62) and Ireland (J. Silke, personal communication), yet only nontoxic isolates have been observed in Norway waters (44). Several nontoxic isolates of G. spinifera have been identified from the United Kingdom (21, 58) and one from the United States (18), yet several toxic isolates from New Zealand (45) and Italy (46) have been observed. Water samples from New Zealand yielded both nontoxic and toxic isolates of G. spinifera, including one isolate that produced the highest amount of YTX per cell for any genus to date (45; L. Rhodes, personal communication). YTX-producing isolates of P. reticulatum have been described from Canada (58), Italy (5), Norway (50), Japan (10, 52), New Zealand (30, 34, 51, 52), Spain (41, 42), South Africa (12, 21), and the United Kingdom (58). Together, these studies indicate that while YTX production by P. reticulatum appears to be globally distributed, a large variation in the toxicity (0.3 to 79 pg YTX cell−1) is observed between isolates of this species. To add to the challenges of establishing monitoring criteria for potential YTX-producing dinoflagellates, there have been conflicting studies published on the toxicity of P. reticulatum from U.S. coastal waters, with reports indicating both the presence and absence of YTX accumulation in cultured isolates from California, Florida, and Washington (41, 42; B. Paz, personal communication).
During environmental YTX events, many dinoflagellates (including species not previously identified to produce YTX) have been isolated, grown in culture, and tested for the production of YTX, particularly members of the Prorocentrum genus which frequently co-occur during these events. This process is labor intensive and dependent on the isolation of a number of cells sufficient for YTX analysis. Given the variation in the expression of toxicity among isolates of the same species and the limited morphological variability within species, we attempted to identify coarse scale genetic markers that would differentiate between spatially and temporally distinct isolates while simultaneously identifying YTX-accumulating strains of L. polyedrum, P. reticulatum, and G. spinifera.
Given the worldwide distribution of potentially toxic dinoflagellates and the global observations of YTX, we speculated that phylogenetic analysis of molecular sequence information from these YTX-producing dinoflagellates could be used as a tool to help identify other potential yet unidentified YTX-producing dinoflagellates. The resultant molecular database could also enhance the subsequent development of robust detection probes for monitoring YTX-producing species.
The use of molecular phylogenetics is now widely utilized in addition to traditional methodologies (such as morphology, ultrastructure, life-cycle information, and fossil record) to understand the evolutionary history of dinoflagellates and to evaluate the relationships within genera among morphologically indistinguishable species. The rRNA operon comprises the genome regions that code for the RNA components of the ribosomes and consists of the large subunit (LSU), the small subunit (SSU), and the 5.8S genes, the latter bound by internal transcribed spacer regions 1 and 2 (ITS1 and ITS2). The rRNA gene has been the target of many phylogenetic studies because ribosomes are universally present in living organisms and functional constraints have resulted in high sequence conservation within these domains (13, 26, 53). The LSU consists of many structural domains (D1 to D12), and the D1, D3, and D8 domains are particularly useful to evaluate the phylogenetic relationships of closely related species (27, 32, 54) since these variable regions flank more conservative regions. The ITS regions are commonly used to analyze closely related and geographically different species (6, 24, 28, 48, 53, 56). The rRNA operon has also been widely and successfully targeted for the design of primers and probes to detect or quantify harmful algal bloom species (2, 31, 32, 55).
MATERIALS AND METHODS
Laboratory cultures.
Cultures were obtained from commercial or individual culture collections. Table 1 lists each species, isolate label name, GenBank accession number, location of isolation, year of isolation, and person or culture collection from which the culture was received. All of the isolates labeled CCMP were received from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton. Only the extracted DNA was received for the L. polyedrum culture CCAP1121/2, obtained from the Culture Collection of Algae and Protozoa (CCAP, Scotland).
TABLE 1.
List of the cultures analyzed, including the label of each, the GenBank accession number, the isolation location, the year of isolation, and the culture collection or person from which the culture was received
| Species | Label | Accession no.
|
Location | Yr | Culture collection | |
|---|---|---|---|---|---|---|
| ITS1-5.8S-ITS2 | LSU D1-D2 | |||||
| Lingulodinium polyedrum | CCMP1931 | EU532481 | EU532472 | Scripps Pier, La Jolla, CA | 1998 | Provasoli-Guillard National Center for Culture of Marine Phytoplankton |
| CCMP1936 | EU532482 | EU532473 | Scripps Pier, La Jolla, CA | 1998 | ||
| 104A | EU532480 | EU532471 | Scripps Pier, La Jolla, CA | 1998 | P. Franks, Scripps Institute of Oceanography | |
| CCAP 1121/2 | EU532483 | EU532474 | Loch Creran, Argyll, Scotland | 1996 | Culture Collection of Algae and Protozoa | |
| Protoceratium reticulatum | CCMP404 | EU532485 | EU532476 | Salton Sea, CA | 1966 | Provasoli-Guillard National Center for Culture of Marine Phytoplankton |
| CCMP1889 | EU532484 | EU532475 | Friday Harbor, San Juan Island, WA | 1983 | ||
| CTCC01 | EU532486 | EU532477 | South Africa | 2006 | G. Pitcher, Marine and Coastal Management, South Africa | |
| Gonyaulax spinifera | CCMP409 | EU532487 | EU532478 | West Boothbay Harbor, MA | 1986 | Provasoli-Guillard National Center for Culture of Marine Phytoplankton |
| Prorocentrum minimum | CCMP1329 | EU532479 | Great South Bay, Long Island, NY | 1958 | Provasoli-Guillard National Center for Culture of Marine Phytoplankton | |
The cultures were grown under nonaxenic conditions in autoclaved glass flasks with either f/2 or L1 medium (15, 16, 17) with seawater stocks obtained from Granite Canyon, California. The culture densities were grown to approximately 103 cells ml−1. The culture conditions were standardized with growth temperatures of 21°C (±1°C) and 87 μmol photons m−2 s−1 irradiance using Sylvania “grow-lite” spectrally corrected light sources on a 14:10 light/dark cycle for cultures CCMP1931, CCMP1936, 104A (all L. polyedrum), and CCMP404 (P. reticulatum). The remaining cultures—CCMP1889 and CTCC01 (P. reticulatum), CCMP409 (G. spinifera), and CCMP1329 (P. minimum)—were grown at 15°C (±1°C), with 70 μmol photons m−2 s−1 irradiance using Sylvania “grow-lite” spectrally corrected light sources on a 12:12 light/dark cycle.
Duplicate samples of each culture were gently filtered onto Poretics, 5.0-μm polycarbonate filters (Osmonics, Inc.), at mid-exponential growth phase. The cells were resuspended in medium and centrifuged in a Fisher Scientific Marathon 8K centrifuge for 3 min at 1,073 × g (4,000 rpm). The supernatant was pipetted off, and the pellets were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
DNA extraction and PCR amplification.
The genomic DNA was extracted from the pellets using NucleoSpin plant kits (BD Biosciences) according to the manufacturer's instructions. Approximately 50 ng of genomic DNA was amplified using 50-μl PCRs containing nuclease-free water (Fisher Scientific), 1× JumpStart PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl; Sigma-Aldrich), 2.5 mM MgCl, 0.2 mM deoxynucleoside triphosphate (Advantage ultrapure PCR deoxynucleotide mix; BD Biosciences), 200 nM of gene-specific oligonucleotide primers, and 0.5 U JumpStart Taq DNA polymerase (Sigma-Aldrich). The ITS1, 5.8S, and ITS2 regions (approximately 800 bp) were amplified from each culture using several primers, each of which is listed in Table 2. The Lp1F and Lp2R oligonucleotide pairs were based on the L. polyedrum ribosomal DNA (rDNA) sequence AF377944 and designed to amplify the entire ITS1-5.8S-ITS2 region from the 3′ end of the SSU rDNA and 5′ end of the LSU rDNA. This dinoflagellate-specific primer set was used successfully to amplify this domain from isolates CCMP404, CCMP1889, and CTCC01 (all P. reticulatum) and CCMP1931, CCMP1936, 104A, and CCAP1121/2 (all L. polyedrum). The more generic oligonucleotide pair 1400F and 38R targeting the same domain (29) were needed to amplify the entire ITS1-5.8S-ITS2 domain from the G. spinifera isolate CCMP409. All amplifications were carried out in duplicate using the Perkin Elmer GeneAmp PCR system 2400 and the following cycling conditions: initial template denaturation for 5 min at 94°C, followed by 33 cycles of 94°C for 30 s, 58°C for 30 s, and extension at 72°C for 1 min and 15 s, followed by a final extension at 72°C for 7 min.
TABLE 2.
List of primers used in the DNA amplification of cultures
A 700-bp fragment of the LSU rDNA, spanning the D1 and D2 regions of the hypervariable domains (26, 27, 54), was amplified using the primers D1R (forward) and D2C (reverse) for all cultures. All amplifications were carried out in duplicate using the Perkin Elmer GeneAmp PCR system 2400 as described above with the annealing temperature decreased to 55°C.
The quality and specificity of all the amplification products were assessed by agarose gel electrophoresis. Individual product bands visualized by staining with ethidium bromide were purified using the Wizard SV gel and PCR clean-up system (Promega) according to the manufacturer's instructions. Products eluted from the solid-phase matrix were further cleaned by isopropanol precipitation. After verifying the purity and concentration by agarose gel electrophoresis, individual template samples were sequenced bidirectionally from the appropriate oligonucleotide primer using a commercial service (Northwoods DNA, Inc.). Consensus sequences obtained for this study were deposited in GenBank using the BankIT facility (Table 1).
Alignment and phylogenetic analysis.
The sequences were aligned using ClustalX, version 1.8 (60), and subsequently adjusted manually. The new ITS and LSU sequences from this study (Table 1) were aligned with existing ITS and LSU sequences available in GenBank (Tables 3 and 4). Phylogenetic relationships were inferred based on the simple pairwise differences of nucleotides (p-distance) derived from the alignment, ignoring gaps. The p-distance represents the number of nucleotide differences divided by the total number of nucleotides compared. The neighbor joining (NJ) distance method and maximum parsimony (MP) estimation of phylogenetic relationships were conducted using the Molecular Evolutionary Genetics Analysis (MEGA) program version 3.1 (23). Bootstrapping values of 1,000 replicates were used to assess the consistency of each derived topology. Due to differences in the availability of sequence datasets for the targeted domains, two outgroups were used separately in these analyses: the dinoflagellate Heterocapsa sp. (GenBank accession number AB084100) was used in the ITS analysis, and the dinoflagellate Symbiodinium microadriaticum (GenBank accession number AF060896) was used in the LSU analysis. These outgroups were selected based on their having the largest p-distance with respect to the YTX-producing taxa.
TABLE 3.
List of sequences used in the ITS region rDNA phylogenetic analysis from GenBank
| Species | Accession no. | Origin |
|---|---|---|
| Akashiwo sanguinea | AY831410 | South Korea |
| AY831411 | South Korea | |
| AY831412 | New York | |
| AB232670 | Japan | |
| Alexandrium catenella | AJ298900 | Spain |
| AJ968683 | Spain | |
| Alexandrium margalefeii | AJ251208 | Italy |
| Gonyaulax spinifera | AF051832 | |
| Heterocapsa sp. | AB084100 | Japan |
| Lingulodinium polyedrum | AF377944 | Korea |
| AM184208 | Italy | |
| Karenia brevis | AF352368 | |
| AF352369 | ||
| Karenia mikimotoi | AM184206 | United Kingdom |
| Karlodinium sp. | AM184205 | Spain |
| Karlodinium micrum | AF352365 | North Carolina |
| AJ557026 | Norway | |
| Prorocentrum micans | AF208245 | |
| Prorocentrum minimum | AF352370 | North Carolina |
| AF208244 | ||
| AF352371 | North Carolina | |
| Protoceratium reticulatum | AM183800 | Italy |
TABLE 4.
List of sequences used in the LSU region rDNA phylogenetic analysis from GenBank
| Species | Accession no. | Origin |
|---|---|---|
| Akashiwo sanguinea | AF260396 | |
| AF260397 | ||
| AY831410 | South Korea | |
| AY831411 | South Korea | |
| AY831412 | New York | |
| Alexandrium catenella | AF200667 | California |
| Alexandrium margalefeii | AY154958 | |
| Alexandrium pseudogonyaulax | AY154957 | |
| Cochlodinium polykrikoides | AY347309 | South Korea |
| AF067861 | ||
| Gonyaulax baltica | AY154962 | |
| Gonyaulax digitale | AY154963 | |
| Gonyaulax elongata | AY154964 | United Kingdom |
| Gonyaulax membranacea | AY154965 | Ireland |
| Gonyaulax polygramma | DQ162802 | Korea |
| Gonyaulax spinifera | DQ151557 | New Zealand |
| DQ151558 | New Zealand | |
| AY154960 | ||
| EF416284 | ||
| Heterocapsa triquetra | AF260401 | Denmark |
| Lingulodinium polyedrum | AF377944 | Korea |
| EF613357 | Korea | |
| Karenia brevis | AY355457 | Florida |
| AY355459 | Florida | |
| AY355458 | Florida | |
| AY355456 | Texas | |
| AY355455 | Texas | |
| Karenia mikimotoi | AY355460 | Florida |
| Karlodinium micrum | AY263964 | Australia |
| AY947666 | New Zealand | |
| DQ898222 | ||
| U92257 | New Zealand | |
| AY947665 | New Zealand | |
| Karlodinium veneficum | DQ114466 | United Kingdom |
| Prorocentrum mexicanum | AJ567468 | |
| AF260378 | ||
| Prorocentrum micans | AY863008 | |
| AF042814 | Korea | |
| AF260377 | Denmark | |
| AY032654 | ||
| Protoceratium reticulatum | AY027907 | South Africa |
| AF260386 | ||
| EF065552 | Italy | |
| EF613362 | ||
| Pyrodinium bahamense var. compressum | AY154959 | The Philippines |
| Symbiodinium microadriaticum | AF060896 |
RESULTS
ITS regions.
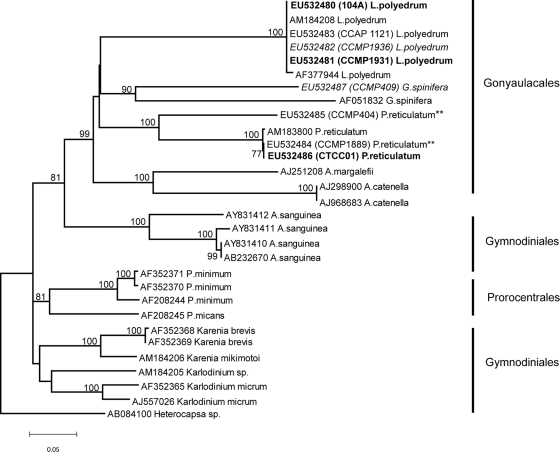
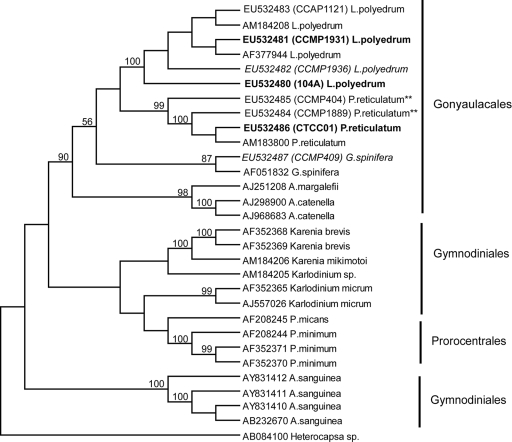
Phylogenetic analysis of the ITS alignments placed all the YTX-producing species identified to date within a monophyletic clade encompassing the dinoflagellate order Gonyaulacales. The resultant topology was well supported (bootstrap values were 99% by NJ analysis [Fig. 1] and 90% by parsimony [Fig. 2]), which agrees with morphologically based taxonomic data. Differences in the intraspecific diversity within the YTX-producing taxa were also revealed by this analysis. There was very low or no diversity among isolates of L. polyedrum. Intraspecific diversity increased among P. reticulatum isolates and was highest among the G. spinifera isolates sequenced to date.
FIG. 1.
NJ analysis of the ITS rDNA for all species listed in Tables 1 and 3. Bootstrap values (1,000 replicates) are listed as percentages of 100, and only values greater than 50% are shown. Heterocapsa sp. (GenBank accession number AB084100) was the outgroup. Cultures of Lingulodinium polyedrum and Protoceratium reticulatum in bold are toxic, and cultures of Lingulodinium polyedrum and Gonyaulax spinifera in italics are nontoxic. Cultures of Protoceratium reticulatum for which there are conflicting studies on toxicity are indicated with double asterisks (**) after the name. The toxicity is unknown for all of the YTX-producing species in the regular font.
FIG. 2.
Maximum parsimony analysis of the ITS rDNA for all species listed in Tables 1 and 3. Bootstrap values (1,000 replicates) are listed as percentages of 100, and only values greater than 50% are shown. Heterocapsa sp. (GenBank accession number AB084100) was the outgroup. Cultures of Lingulodinium polyedrum and Protoceratium reticulatum in bold are toxic, and cultures of Lingulodinium polyedrum and Gonyaulax spinifera in italics are nontoxic. Cultures of Protoceratium reticulatum for which there are conflicting studies on toxicity are indicated with double asterisks (**) after the name. The toxicity is unknown for all of the YTX-producing species in the regular font.
For the rRNA loci sequenced for this study, alignments revealed that there was no intraspecific genetic diversity (0.000 p-distance) among the known isolates of L. polyedrum isolates. However, a comparison of these sequences to a GenBank record for this species (AF377944) revealed two separate nucleotide differences (0.006 p-distance) in the ITS1 region, each representing a transition. There was no genetic divergence (0.000 p-distance) between the P. reticulatum culture isolated from Washington (EU532484, culture CCMP1889) and the toxic South Africa isolate (EU532486, culture CTCC01); however, the culture from the Salton Sea (EU532485, CCMP404), a saline lake in California, was highly divergent (0.235 p-distance) from the two coastal ocean isolates. The Washington and California cultures have been reported to be toxic (41) and nontoxic (42; B. Paz, personal communication), respectively. For the purposes of this study, we will consider the toxicity of these cultures uncertain. The GenBank sequence AM183800 was only slightly divergent (0.003 p-distance) from the coastal ocean isolates (EU532484, culture CCMP1889, and EU532486, culture CTCC01) and more divergent (0.235 p-distance) from the saline lake isolate (EU532485, culture CCMP404). There were only two G. spinifera isolates used in the ITS analysis (due to a lack of sequence information for the ITS region in GenBank). These two isolates were genetically distinct (0.350 p-distance), and the toxicity was known for one of those sequences (EU532487, CCMP409).
LSU region.
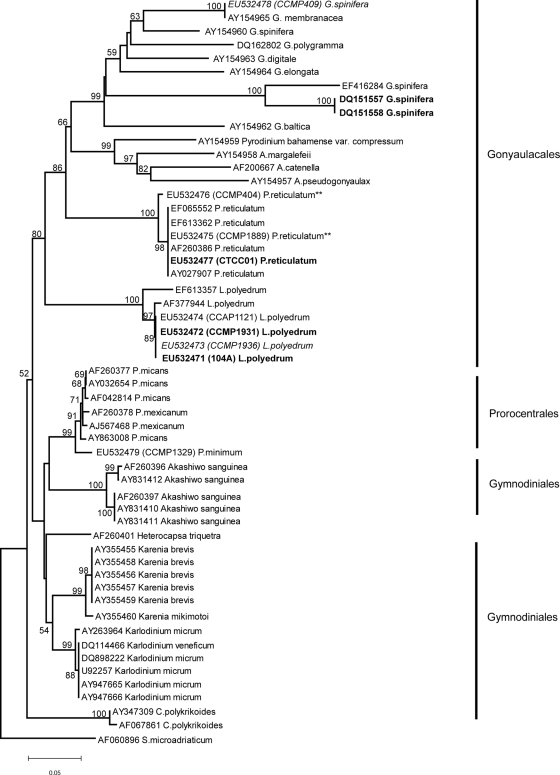
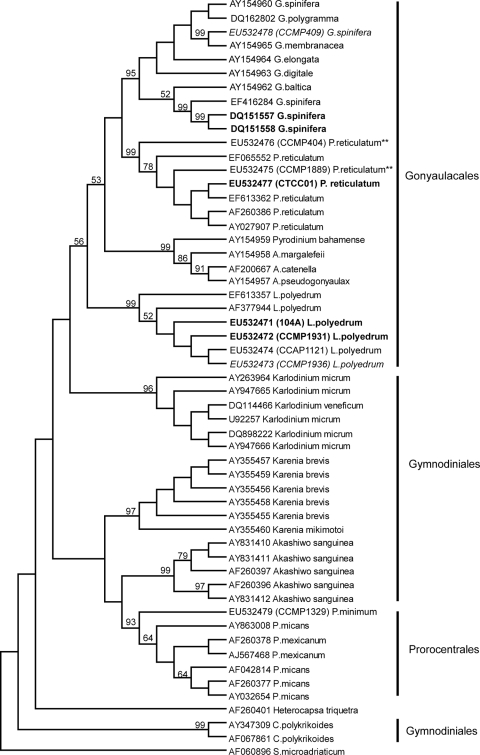
In both the NJ (Fig. 3) and maximum parsimony (Fig. 4) analyses of the LSU domains D1 and D2, the Gonyaulacales order again formed a distinct clade, although with lower support (bootstrap values 80% and 56%, respectively) compared with the ITS analyses.
FIG. 3.
NJ analysis of the LSU rDNA for all species listed in Tables 1 and 4. Bootstrap values (1,000 replicates) are listed as percentages of 100, and only values greater than 50% are shown. Symbiodinium microadriaticum (GenBank accession number AF060896) was the outgroup. Cultures of Lingulodinium polyedrum, Protoceratium reticulatum, and Gonyaulax spinifera in bold are toxic and cultures of Lingulodinium polyedrum and Gonyaulax spinifera in italics are nontoxic. Cultures of Protoceratium reticulatum for which there are conflicting studies on toxicity are indicated with double asterisks (**) after the name. The toxicity is unknown for all of the YTX-producing species in the regular font.
FIG. 4.
Maximum parsimony analysis of the LSU rDNA for all species listed in Tables 1 and 4. Bootstrap values (1,000 replicates) are listed as percentages of 100, and only values greater than 50% are shown. Symbiodinium microadriaticum (GenBank accession number AF060896) was the outgroup. Cultures of Lingulodinium polyedrum, Protoceratium reticulatum, and Gonyaulax spinifera in bold are toxic, and cultures of Lingulodinium polyedrum and Gonyaulax spinifera in italics are nontoxic. Cultures of Protoceratium reticulatum for which there are conflicting studies on toxicity are indicated with double asterisks (**) after the name. The toxicity is unknown for all of the YTX-producing species in the regular font.
As in the ITS analysis, molecular discrimination among geographically distinct isolates of L. polyedrum was not detected (0.000 p-distance). However, this LSU domain exhibited more variability among the strains from the GenBank database. While the Korean isolate described in GenBank (AF377944) had a similar level of divergence as that observed for the L. polyedrum cultures sequenced in this study (0.006 p-distance), this divergence was due to only 3 nucleotide differences in the sequences, likely due to sequencing errors. Another submission again from Korean waters (EF613357) exhibited greater divergence at this locus (0.041 p-distance). While there was no genetic difference (0.000 p-distance) among the coastal ocean P. reticulatum cultures, there was a slight difference (0.0013 p-distance) between these isolates and the saline lake isolate (EU532476), which was consistent with the ITS analyses. The LSU sequences from GenBank were genetically identical to the coastal P. reticulatum cultures sequenced in this study.
The sequence diversity was considerably higher among isolates designated as G. spinifera. Interestingly, the two toxin-producing strains of G. spinifera, DQ151557 and DQ151558 (45), were genetically identical to each other (0.000 p-distance) but distinct from the non-toxin-producing isolate EU532478 (0.330 p-distance) and GenBank strain AY154960 (0.3 p-distance), toxicity unknown. The toxic strains were less divergent from GenBank strain EF416284 (0.130 p-distance), toxicity unknown. The GenBank submission assigned as G. membranacea (AY154965) was found to be identical to the LSU sequence derived from the G. spinifera strain (CCMP409) used in this study, indicating an incorrect species annotation either in the original submission or in the CCMP culture collection.
DISCUSSION
The evolution of dinoflagellates has been intensely studied, and it has become common to use traditional methodologies, such as thecal plate and whole-cell morphology, ultrastructure, life cycles, and fossil record, in combination with molecular phylogenetics (13, 59). The dinoflagellates consist of six major groups, Gymnodiniales, Gonyaulacales, Prorocentrales, Peridiniales, Dinophysiales, and Suessiales (59). While most dinoflagellates have been classified according to morphological characteristics, a range of observations indicate a consistent pattern of differences in phycotoxicity within morphologically identical species. The potential for molecular sequence information to provide additional higher-resolution discriminatory characters may help our understanding of the evolutionary differences and monitoring of bloom dynamics between isolates of the same species.
There are a large number of studies that have used nuclear rRNA genes successfully to evaluate relationships among closely related taxa within genera and particularly within species that are morphologically indistinguishable using the variable domains within the LSU region (27, 29, 32, 54) and potentially more rapidly evolving ITS region (6, 24, 28, 48, 53, 56). While a consensus molecular phylogeny has not been accepted for the Dinophyceae as a whole, there are broad consistencies in the topological positions of some taxonomic orders within this group. For example, previous dinoflagellate phylogeny studies have found the Gonyaulacales order to be a monophyletic and more recently diverged group relative to other described orders within the Dinophyceae (37, 63).
In all phylogenetic analyses in this study, the Gonyaulacales order formed a distinct clade, consistent with previously published studies (7, 13, 37, 49, 59, 63). The molecular sequence analysis for the two loci examined in this study support the validity of the Gonyaulacales and firmly place all known YTX-producing species within this dinoflagellate order. The Prorocentrales order was included in these analyses because Prorocentrum micans was frequently present when YTX was detected in California mussels samples (18; M. Silver, unpublished data), and several isolates from the United Kingdom have been tested for YTX production (58). The results of this study, however, suggest that non-Gonyaulacaloid dinoflagellate species (such as Prorocentrum) are unlikely to produce YTX, and testing such species that fall outside of the Gonyaulacales order may be ineffective for identifying the biological origin of YTX during events.
The L. polyedrum species showed very low intraspecific diversity, and at this level of genetic assessment, there did not appear to be geographically distinct populations but rather a global distribution of ribotypes within this species. Consequently, rRNA operons provide no indirect genetic markers for YTX production, and lack of diversity at this level hints that variation in toxicity may be due to environmental conditions or genomic variability. Toxin production in other algae, such as Alexandrium spp., P. reticulatum, and Pseudo-nitzschia spp., has been shown to be influenced by environmental conditions, particularly nutrients (1, 4, 14, 19, 38, 39, 40), although under common culture conditions, some isolate-specific variation can be maintained (22, 57). Future studies should evaluate if there are specific genes that are “turned on” during toxin production (42), which would explain the differences between toxic and nontoxic isolates of the same, genetically similar species. The lack of genetic diversity indicates that overall quantification may be the most effective methodology in toxic bloom monitoring of L. polyedrum. A quantitative PCR method based on the SSU has been developed for the quantification of L. polyedrum from southern California (35). The results from this study of the LSU and ITS regions of L. polyedrum populations suggest that the SSU quantitative PCR method can be applied to other geographic regions to aid in monitoring L. polyedrum bloom dynamics.
There was a large sequence divergence observed between multiple isolates in the LSU analyses of G. spinifera, which is consistent with other studies of the LSU region of the Gonyaulax genus (11, 20, 45, 46). While a limited number of LSU sequences were used in this study, distinct ribotypes of toxic G. spinifera (DQ151557 and DQ151558) were revealed. In a similar phylogenetic analysis of the LSU region, toxic strains of G. spinifera from New Zealand (DQ151557 and DQ151558) (45) and Italy (46) grouped together and formed a clade distinct from other strains and had a high intraspecific variability compared with GenBank strains (46). The high levels of intraspecific genetic divergence detected in our analysis (30 to 40%) as well as from Riccardi et al. (46) suggest that G. spinifera is undergoing rapid diversification. Considerable morphological variability is observed for G. spinifera (8, 61), and it is possible that cryptic species may exist within the known isolates of G. spinifera. Notwithstanding current taxonomic assignments, the members of the genus Gonyaulax may provide fruitful targets for the development of molecular probes distinguishing isolates of differing toxicity and for the examination of how the expression of toxin production may drive the evolution or diversification of harmful algal species. Additional multilocus and ultrastructural studies using a large number of unique G. spinifera isolates will be needed to thoroughly describe the genetic variability within this species.
Due to the conflicting reports of toxicity for P. reticulatum cultures CCMP404 and CCMP1889, it is difficult to determine if there are genetically distinguishable isolates of this species based on toxicity. However, there are several published studies evaluating the influence of environmental conditions on the production of YTX in P. reticulatum. In isolates of P. reticulatum from Emilia-Romagna, Italy, YTX production increased within the cells when cultures were grown under higher salinity and temperature conditions and under both replete and severe phosphate-limited nutrient conditions (14). YTX released from cells into the medium was found to be higher in cultures grown under nitrogen limitation, lower temperature or lower salinity conditions. Those authors concluded that environmental conditions directly affect toxin production and that decreased temperature and salinity will decrease toxin production but will not terminate toxin production in cells (14). In culture, the stationary growth phase, as well as the addition of the micronutrient selenium but not iron or cobalt, significantly increased YTX production by P. reticulatum (33, 34). These published studies suggest that environmental conditions can influence toxicity and therefore that genetically distinct isolates based on toxicity may not exist. Future research on this species will need to evaluate the genetic diversity of strains for which a complete toxin profile has already been established, such as the isolated cultures from Spain (42).
We hypothesized that the rRNA genes may be useful for the evaluation of genetic variability among toxic and nontoxic isolates. However, the results of this study show that the constrained sequence variability of several rRNA operons do not provide robust markers of YTX toxicity among species for most genera in the Gonyaulacales order. While this study and the results of Riccardi et al. (46) suggest that this application might be possible for G. spinifera, these results are based on small sets of isolates, and therefore, a larger number of G. spinifera isolates needs to be analyzed to characterize the true genetic and toxicity associations within this species. If Gonyaulax is indeed undergoing rapid diversification, this genus may be suitable for genomic tools, but we suggest that future studies focus on both physiological and genomic assays for YTX production in Gonyaulacales. Our results do demonstrate, however, that confirmed YTX production is currently confined to the order Gonyaulacales within the Dinophyceae and that species within this taxonomic order should be given priority for future testing and field collections associated with monitoring for YTX contamination events. Interestingly, a previous study evaluating the origin of paralytic shellfish poisoning (PSP) toxins concluded that PSP toxin-producing species are randomly distributed throughout all dinoflagellate groups and, based on this widespread toxin distribution, speculated that PSP toxins had multiple independent origins in the Gymnodiniales and Gonyaulacales orders (63). While other species within the Gonyaulacales order have not been documented to produce YTX congeners (e.g., Alexandrium catenella), all YTX-producing species identified so far remain within this group. Our results contrast with this hypothesis of multiple origins for PSP toxins, as the occurrence of YTX production within several distinct genera in the Gonyaulacales support the hypothesis that YTX biosynthetic capacity arose early in the divergence of this order and consequently later in the evolutionary history of the Dinophyceae.
Acknowledgments
This work was supported by the University of California Coastal Environmental Quality Initiative Program award 05TCEQI070075 awarded to R.M.K. and M.D.A.H. and other grants awarded to M.D.A.H., including the Ida Benson Lynn fellowship, the Marilyn C. Davis memorial scholarship, the University of California, the Santa Cruz Women's Club, and the Ocean Science Department at the University of California, Santa Cruz. G.J.S. acknowledges prior NSF support (OCE-0138547) for molecular facilities.
We thank Bethany Jenkins and Kendra Hayashi. We thank the Marine Pollution Studies Laboratory at Granite Canyon, University of California, Davis, for the seawater they provided, and Grant Pitcher and Peter Franks for providing algal strains used in this study. We also thank three anonymous reviewers who provided useful commentary on an earlier version of the manuscript.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Anderson, D. M., D. M. Kulis, J. J. Sullivan, S. Hall, and C. Lee. 1990. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Mar. Biol. 104:511-524. [Google Scholar]
- 2.Anderson, D. M. 1995. Identification of harmful algal species using molecular probes: an emerging perspective, p. 3-13. In P. Lassus, G. Arzul, P. Gentien, and C. Marcaillou (ed.), Harmful marine algal blooms. Lavoisier, Paris, France.
- 3.Armstrong, M., and R. Kudela. 2006. Evaluation of California isolates of Lingulodinium polyedrum for the production of yessotoxin. Afr. J. Mar. Sci. 28:399-401. [Google Scholar]
- 4.Armstrong Howard, M. D., W. P. Cochlan, R. M. Kudela, N. Ladizinsky, and R. M. Kudela. 2007. Nitrogenous preference of toxic Pseudo-nitzschia australis (Bacillariophyceae) from field and laboratory experiments. Harmful Algae 6:206-217. [Google Scholar]
- 5.Boni, L., A. Ceredi, F. Guerrini, A. Milandri, R. Pistocchi, R. Poletti, and M. Pompei. 2002. Toxic Gonyaulax Grindley Reinecke in the north-western Adriatic Sea (Italy). Abstr. 10th Int. Harmful Algal Bloom Conf., St. Petersburg, FL.
- 6.Coyer, J. A., G. J. Smith, and R. A. Andersen. 2001. Evolution of Macrocystis spp. (Phaeophyceae) as determined by ITS1 and ITS2 sequences. J. Phycol. 37:574-585. [Google Scholar]
- 7.Daugbjerg, N., G. Hansen, J. Larsen, and Ø. Moestrup. 2000. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmored dinoflagellates. Phycologia 39:302-317. [Google Scholar]
- 8.Dodge, J. D. 1989. Some revisions of the family Gonyaulacaceae (Dinophyceae) based on scanning electron microscope study. Bot. Mar. 32:275-298. [Google Scholar]
- 9.Draisci, R., E. Ferretti, L. Palleschi, C. Marchiafava, R. Poletti, A. Milandri, A. Ceredi, and M. Pompei. 1999. High levels of yessotoxin in mussels and presence of yessotoxin and homoyessotoxin in dinoflagellates of the Adriatic Sea. Toxicon 37:1187-1193. [DOI] [PubMed] [Google Scholar]
- 10.Eiki, K., M. Satake, K. Koike, T. Ogata, T. Mitsuya, and Y. Oshima. 2005. Confirmation of yessotoxin production by the dinoflagellate Protoceratium reticulatum in Mutsu Bay. Fish. Sci. 71:633-638. [Google Scholar]
- 11.Ellegaard, M., N. Daugbjerg, A. Rochon, J. Lewis, and I. Harding. 2003. Morphological and LSU rDNA sequence variation within the Gonyaulax spinifera-Spiniferites group (Dinophyceae) and proposal of G. elongata comb. nov. and G. membranacea comb. nov. Phycologia 42:151-164. [Google Scholar]
- 12.Fawcett, A., G. C. Pitcher, S. Bernard, A. D. Cembella, and R. M. Kudela. 2007. Contrasting wind patterns and toxigenic phytoplankton in the southern Benguela upwelling system. Mar. Ecol. Prog. Ser. 348:19-31. [Google Scholar]
- 13.Fensome, R. A., J. F. Saldarriaga, and F. J. R. Taylor. 2000. Dinoflagellate phylogeny revisited: reconciling morphological and molecular based phylogenies. Grana 38:66-80. [Google Scholar]
- 14.Guerrini, F., P. Ciminiello, C. Dell'Aversano, L. Tartaglione, E. Fattorusso, L. Boni, and R. Pistocchi. 2007. Influence of temperature, salinity and nutrient limitation on yessotoxin production and release by the dinoflagellate Protoceratium reticulatum in batch cultures. Harmful Algae 6:707-717. [Google Scholar]
- 15.Guillard, R. R. L., and J. H. Ryther. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 8:229-239. [DOI] [PubMed] [Google Scholar]
- 16.Guillard, R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 26-60. In W. L. Smith and M. H. Chanely (ed.), Culture of marine invertebrate animals. Plenum Press, New York, NY.
- 17.Guillard, R. R. L., and P. E. Hargraves. 1993. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 32:234-236. [Google Scholar]
- 18.Howard, M. D. A., M. Silver, and R. M. Kudela. 2008. Yessotoxin detected in mussel (Mytilus californicus) and phytoplankton samples from the U.S. west coast. Harmful Algae 7:646-652. [Google Scholar]
- 19.Hwang, D. F., and Y. H. Lu. 2000. Influence of environmental and nutritional factors on growth, toxicity and toxin profile of dinoflagellate Alexandrium minutum. Toxicon 38:1491-1503. [DOI] [PubMed] [Google Scholar]
- 20.Kim, K.-Y., Y.-S. Kim, C.-H. Hwang, C.-K. Lee, W.-A. Lim, and C.-H. Kim. 2006. Phylogenetic analysis of dinoflagellate Gonyaulax polygramma Stein responsible for harmful algal blooms based on the partial LSU rDNA sequence data. Algae 21:1-10. [Google Scholar]
- 21.Krock, B., T. Alpermann, U. Tilmann, G. C. Pitcher, and A. D. Cembella. 2006. Yessotoxin profiles of marine dinoflagellates Protoceratium reticulatum and Gonyaulax spinifera, p. 303-305. In O. Moestrup (ed.), Proceedings of the XII International Conference on Harmful Algae. International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission, Copenhagen, Denmark, 4 to 8 September 2006.
- 22.Kudela, R. M., A. Roberts, and M. Armstrong. 2003. Laboratory analyses of nutrient stress and toxin production in Pseudo-nitzschia spp. from Monterey Bay, California, p. 136-138. In K. A. Steidinger, J. H. Landsberg, C. R. Tomas, and G. A. Vargo (ed.), Harmful algae 2002. Florida and Wildlife Conservation Commission, Florida Institute of Oceanography, and Commission of UNESCO, St. Petersburg, FL.
- 23.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 24.LaJeunesse, T. C. 2001. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J. Phycol. 37:866-880. [Google Scholar]
- 25.Reference deleted.
- 26.Lenaers, G., L. Maroteaux, B. Michot, and M. Herzog. 1989. Dinoflagellates in evolution. A molecular phylogenetic analysis of large subunit ribosomal RNA. J. Mol. Evol. 29:40-51. [DOI] [PubMed] [Google Scholar]
- 27.Lenaers, G., C. Scholin, Y. Bhaud, D. Saint-Hilaire, and M. Herzog. 1991. A molecular phylogeny of dinoflagellate protests (Pyrrhophyta) inferred from the sequence of 24S rRNA divergent domains D1 and D8. J. Mol. Evol. 32:53-63. [DOI] [PubMed] [Google Scholar]
- 28.Litaker, R. W., M. W. Vandersea, S. R. Kibler, K. S. Reece, N. A. Stokes, F. M. Lutzoni, B. A. Yonish, M. A. West, M. N. D. Black, and P. A. Tester. 2007. Recognizing dinoflagellate species using ITS rDNA sequences. J. Phycol. 43:344-355. [Google Scholar]
- 29.Lundholm, N., Ø. Moestrup, G. R. Hasle, and K. Hoef-Emden. 2003. A study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): what is P. pseudodelicatissima? J. Phycol. 39:797-813. [Google Scholar]
- 30.MacKenzie, L., P. Holland, P. McNabb, V. Beuzenberg, A. Selwood, and T. Suzuki. 2002. Complex toxin profiles in phytoplankton and greenshell mussels (Perna canaliculus), revealed by LC-MS/MS analysis. Toxicon 40:1321-1330. [DOI] [PubMed] [Google Scholar]
- 31.Mikulski, C. M., S. L. Morton, and G. J. Doucette. 2005. Development and application of LSU rRNA probes for Karenia brevis in the Gulf of Mexico, USA. Harmful Algae 4:49-60. [Google Scholar]
- 32.Miller, P. E., and C. A. Scholin. 1996. Identification of culture Pseudo-nitzschia (Bacillariophyceae) using species-specific LSU rRNA targeted fluorescent probes. J. Phycol. 32:646-655. [Google Scholar]
- 33.Mitrovic, S. M., M. Fernández Amandi, L. MacKenzie, A. Furey, and K. J. James. 2004. Effects of selenium, iron and cobalt addition to growth and yessotoxin production of the toxic marine dinoflagellate Protoceratium reticulatum in culture. J. Exp. Mar. Biol. Ecol. 313:337-351. [Google Scholar]
- 34.Mitrovic, S. M., B. Hamilton, L. MacKenzie, A. Furey, and K. J. James. 2005. Persistence of yessotoxin under light and dark conditions. Mar. Environ. Res. 60:397-401. [DOI] [PubMed] [Google Scholar]
- 35.Moorthi, S. D., P. D. Countway, B. A. Stauffer, and D. A. Caron. 2006. Use of quantitative real-time PCR to investigate the dynamics of the red tide dinoflagellate Lingulodinium polyedrum. Microb. Ecol. 52:136-150. [DOI] [PubMed] [Google Scholar]
- 36.Murata, M., M. Kumagai, J. S. Lee, and T. Yasumoto. 1987. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 28:5869-5872. [Google Scholar]
- 37.Murray, S., M. F. Jørgensen, S. Y. W. Ho, D. J. Patterson, and L. S. Jermiin. 2005. Improving the analysis of dinoflagellate phylogeny based on rDNA. Protist 156:269-286. [DOI] [PubMed] [Google Scholar]
- 38.Pan, Y., D. V. Subba Rao, K. H. Mann, R. G. Brown, and R. Pocklington. 1996. Effects of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. I. Batch culture studies. Mar. Ecol. Prog. Ser. 131:225-233. [Google Scholar]
- 39.Pan, Y., D. V. Subba Rao, K. H. Mann, W. K. W. Li, and W. G. Harrison. 1996. Effects of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. II. Continuous culture studies. Mar. Ecol. Prog. Ser. 131:235-243. [Google Scholar]
- 40.Pan, Y., D. V. Subba Rao, and K. H. Mann. 1996. Changes in domoic acid production and cellular chemical composition of the toxigenic diatom Pseudo-nitzschia multiseries under phosphate limitation. J. Phycol. 32:371-381. [Google Scholar]
- 41.Paz, B., P. Riobo, M. L. Fernández, S. Fraga, and J. M. Franco. 2004. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 44:251-258. [DOI] [PubMed] [Google Scholar]
- 42.Paz, B., P. Riobó, I. Ramilo, and J. M. Franco. 2007. Yessotoxins profile in strains of Protoceratium reticulatum from Spain and USA. Toxicon 50:1-17. [DOI] [PubMed] [Google Scholar]
- 43.Plumley, F. G. 1997. Marine algal toxins: biochemistry, genetics, and molecular biology. Limnol. Oceanogr. 42:1252-1264. [Google Scholar]
- 44.Ramstad, H., P. Hovgaard, T. Yasumoto, S. Larsen, and T. Aune. 2001. Monthly variations in diarrhetic toxins and yessotoxin in shellfish from coast to the inner part of the Sognefjord, Norway. Toxicon 39:1035-1043. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes, L., P. McNabb, M. de Salas, L. Briggs, V. Beuzenberg, and M. Gladstone. 2005. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 5:148-155. [Google Scholar]
- 46.Riccardi, M., F. Guerrini, F. Roncarati, A. Milandri, M. Cangini, S. Pigozzi, E. Riccardi, A. Ceredi, P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, L. Tartaglione, and R. Pistocchi. Gonyaulax spinifera from the Adriatic Sea: toxin production and phylogenetic analysis. Harmful Algae, in press.
- 47.Riobo, P., B. Paz, M. Fernandez, S. Fraga, and J. M. Franco. 2002. Lipophylic toxins of different strains of Ostreopsidaceae and Gonyaulacaceae. Abstr. 10th Int. Harmful Algal Bloom Conf., St. Petersburg, FL.
- 48.Saito, K., T. Drgon, J. A. F. Robledo, D. N. Krupatkina, and G. R. Vasta. 2002. Characterization of the rRNA locus of Pfiesteria piscicida and development of standard and quantitative PCR-based detection assays targeted to the nontranscribed spacer. Appl. Environ. Microbiol. 68:5394-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saldarriaga, J. R., F. J. R. Taylor, T. Cavalier-Smith, S. Menden-Deuer, and P. J. Keeling. 2004. Molecular data and the evolutionary history of dinoflagellates. Eur. J. Protistol. 40:85-111. [Google Scholar]
- 50.Samdal, I. A., L. J. Naustvoll, C. D. Olseng, L. R. Briggs, and C. O. Miles. 2004. Use of ELISA to identify Protoceratium reticulatum as a source of yessotoxin in Norway. Toxicon 44:75-82. [DOI] [PubMed] [Google Scholar]
- 51.Satake, M., K. Terawawa, Y. Kadowaki, and T. Yasumoto. 1996. Relative configuration of yessotoxin and isolation of two new analogs from toxic scallops. Tetrahedron Lett. 37:5955-5958. [Google Scholar]
- 52.Satake, M., T. Ichimura, K. Sekiguchi, S. Yoshimatsu, and Y. Oshima. 1999. Confirmation of yessotoxin and 45, 46, 47-trinoryessotoxin production by Protoceratium reticulatum collected in Japan. Nat. Toxins 7:147-150. [DOI] [PubMed] [Google Scholar]
- 53.Schlötterer, C. 1998. Ribosomal DNA probes and primers, p. 267-276. In A. Karp, P. G. Isaac, and D. S. Ingram (ed.), Molecular tools for screening biodiversity. Chapman & Hall, London, United Kingdom.
- 54.Scholin, C. A., M. Herzog, M. L. Sogin, and D. M. Anderson. 1994. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 30:999-1011. [Google Scholar]
- 55.Scholin, C. A., R. Marin, III, and P. E. Miller. 1996. DNA probe-based assays for rapid detection of toxic algal species in environmental samples. J. Phycol. 32(Suppl.):43. [Google Scholar]
- 56.Shao, P., Y.-Q. Chen, H. Zhou, J. Yuan, L.-H. Qu, D. Zhao, and Y.-S. Lin. 2004. Genetic variability in Gymnodiniaceae ITS regions: implications for species identification and phylogenetic analysis. Mar. Biol. 144:215-224. [Google Scholar]
- 57.Smith, G. J., N. L. Ladizinsky, and P. E. Miller. 2001. Amino acid profiles in species and strains of Pseudo-nitzschia from Monterey Bay, California: insights into the metabolic role(s) of domoic acid, p. 324-327. In G. M. Hallegraeff, S. I. Blackburn, C. J. Bolch, and R. J. Lewis (ed.), Harmful algal blooms 2000. IOC/UNESCO, Paris, France.
- 58.Stobo, L. A., J. Lewis, M. A. Quilliam, W. R. Hardstaff, S. Gallacher, L. Webster, E. A. Smith, and M. McKenzie. 2003. Detection of yessotoxin in UK and Canadian isolates of phytoplankton and optimization and validation of LC-MS methods. Can. Tech. Rep. Fish. Aquat. Sci. 2498:8-14. [Google Scholar]
- 59.Taylor, F. J. R. 2004. Illumination of confusion? Dinoflagellate molecular phylogenetic data viewed from a primarily morphological standpoint. Phycol. Res. 52:308-324. [Google Scholar]
- 60.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomas, C. R. 1997. Identifying marine phytoplankton, p. 858. Academic Press, San Diego, CA.
- 62.Tubaro, A., L. Sidari, R. Della Loggia, and T. Yasumoto. 1998. Occurrence of yessotoxin in phytoplankton and mussels from northern Adriatic Sea, p. 470-472. In B. Requera, J. Blanco, M. L. Fernández, and T. Wyatt (ed.), Harmful algae. Proceedings of the VIII International Conference on Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO, Vigo, Spain.
- 63.Zardoya, R., E. Costas, V. López-Rodas, A. Garrido-Pertierra, and J. M. Bautista. 1995. Revised dinoflagellate phylogeny inferred from molecular analysis of large-subunit ribosomal RNA gene sequences. J. Mol. Evol. 41:637-645. [DOI] [PubMed] [Google Scholar]