Abstract
To control the antibiotic resistance epidemic, it is necessary to understand the distribution of genetic material encoding antibiotic resistance in the environment and how anthropogenic inputs, such as wastewater, affect this distribution. Approximately two-thirds of antibiotics administered to humans are β-lactams, for which the predominant bacterial resistance mechanism is hydrolysis by β-lactamases. Of the β-lactamases, the TEM family is of overriding significance with regard to diversity, prevalence, and distribution. This paper describes the design of DNA probes universal for all known TEM β-lactamase genes and the application of a quantitative PCR assay (also known as Taqman) to quantify these genes in environmental samples. The primer set was used to study whether sewage, both treated and untreated, contributes to the spread of these genes in receiving waters. It was found that while modern sewage treatment technologies reduce the concentrations of these antibiotic resistance genes, the ratio of blaTEM genes to 16S rRNA genes increases with treatment, suggesting that bacteria harboring blaTEM are more likely to survive the treatment process. Thus, β-lactamase genes are being introduced into the environment in significantly higher concentrations than occur naturally, creating reservoirs of increased resistance potential.
Antibiotic resistance has been classified by the World Health Organization as one of the three major public health threats of the 21st century (81). Essentially all previous efforts to control and study the epidemic have focused on pathogens in the clinical setting. However, antibiotic resistance genes can also occur in nonpathogenic bacteria, which can then be passed on via lateral gene transfer (41, 42). It is thus necessary to understand the environmental distribution of such genes and how anthropogenic inputs, such as wastewater, affect their spread. The availability of sequences obtained from clinical data facilitates the development of functional gene probes and primers, which can be used to quantify families of antibiotic resistance genes among both culturable and unculturable bacteria in environmental samples. Designing probes to be general, rather than specific, allows them to pick up all known variants of the gene and facilitates detection across species. This more comprehensive monitoring strategy is likely to lead to more effective control of the antibiotic resistance epidemic.
The study described here focuses on a family of genes encoding resistance to β-lactam antibiotics. β-Lactams account for approximately two-thirds, by weight, of all antibiotics administered to humans and include the penicillins, the cephalosporins, and the carbapenems (reviewed in reference 38). The predominant mechanism for resistance to β-lactam antibiotics by bacterial pathogens is the production of β-lactamases, which break down β-lactams by hydrolyzing the four-membered β-lactam ring (34, 49).
Even though β-lactamases are estimated to have existed for the past 2 billion years, their evolution and spread have been highly correlated to the anthropogenic development and prolificacy of β-lactam antibiotics during the past 60 years (26). For example, in one British hospital the incidence of β-lactamase-producing Staphylococcus aureus rose from less than 8% to almost 60% in a 4- to 5-year period immediately following World War II, as administration of penicillin became routine practice (4). By the late 1970s, after the discovery of cephalosporin C, other cephalosporin analogs, and broad-spectrum penicillins such as ampicillin, epidemiological studies reported the broad-spectrum TEM β-lactamases to be the most prevalent and widely distributed of plasmid-mediated enzymes (34, 48).
While the production of β-lactamases is particularly common among gram-negative bacteria, they have been identified in virtually all bacterial species, with notable exceptions being most enterococci and salmonellae (8, 30, 61). Although β-lactamase genes are thought to have originally resided solely on bacterial chromosomes, they are often found on plasmids in specimens collected after the introduction of clinical β-lactam antibiotics. The mobility of these genes is related to their association with transposons and even integrons. Transposons have been implicated in the spread of TEM β-lactamase to plasmids in Haemophilus influenzae and Neisseria gonorrhoeae (21, 22). β-Lactamase genes can also be found as components of multiresistance transposons and on multiple plasmids within a given organism (11, 34, 40, 48). In light of specimens that harbor multiple types of β-lactamase genes and the transferability of these genes between species via mobile genetic elements, the idea that β-lactamase genes are species specific is now an old one (11, 34, 60, 82).
The TEM β-lactamases represent one of the most clinically significant families of β-lactamases. The first in this group to be discovered, TEM-1, is considered broad spectrum and hydrolyzes the early cephalosporins, in addition to many penicillins. TEM-1 has become the most commonly encountered β-lactamase and is ubiquitous among Enterobacteriaceae (48, 49). TEM-3 was the first of the extended-spectrum β-lactamases (ESBLs), which have an increased substrate spectrum, including third-generation cephalosporins, but are susceptible to β-lactamase inhibitors such as clavulanic acid. The majority of TEM types discovered subsequently are ESBLs. However, some TEM β-lactamases are inhibitor resistant, and TEM types are now being discovered that are both ESBLs and inhibitor resistant. A few select point mutations, within the approximately 75 mutations that distinguish specific TEM enzymes, are responsible for these general phenotypes (11).
To our knowledge, quantitative real-time PCR probes universal for all known variations of TEM β-lactamase genes did not previously exist. Such a primer set was developed in order to study the concentration of TEM β-lactamase genes along a wastewater stream and to determine whether wastewater contributes to their proliferation in receiving waters. Little is known about the connection between wastewater and antibiotic resistance, yet many characteristics of wastewater make it a highly suspect medium of spread, i.e., the presence of antibacterials from household products (soaps, detergents, etc.), the presence of antibiotics that have been excreted from humans or disposed of down a drain, and a high bacterial load. By using very sensitive instrumentation to quantify DNA concentration and universal primers that reliably amplify the 16S small subunit rRNA gene, it was possible to normalize the β-lactamase gene counts to proxies of biomass and bacterial count, respectively (73).
The discharge from the Deer Island treatment plant (DITP) into Massachusetts Bay provided a model system to study the dispersal of β-lactamase genes. The DITP receives sewage from Boston and its surrounding communities, from both residential and institutional sources, including hospitals. Completed in 2001, the plant utilizes some of the most modern treatment technologies, including primary and secondary treatment, chlorination, dechlorination, and anaerobic sludge digestion. DITP's effluent is transported offshore via a 15-km tunnel and dispelled through a diffuser system, consisting of 55 diffuser caps in the seafloor spread along the last 2 km of the tunnel's length. Aside from the potential spread of antibiotic resistance, the input of sewage into Massachusetts Bay has been studied extensively, both in terms of characterizing the plume and measuring the ecological effects. Currents acting on the plume arise from the general pattern of inflow from the Gulf of Maine and secondary effects of wind, density distribution, and local mixing, all of which have been described in detail (53). Six years of monitoring data (in addition to nearly 9 years of baseline data collected prior to the completion of the current outfall) and a dye dilution study allowed us to identify sites that consistently lie within the densest regions of the plume and others that are not measurably affected by it (53). Because so little is known about the dispersal of β-lactamase genes in the environment, it was important to choose such a well-characterized system, so that anthropogenic effects could be distinguished with confidence.
MATERIALS AND METHODS
Collection and processing of samples.
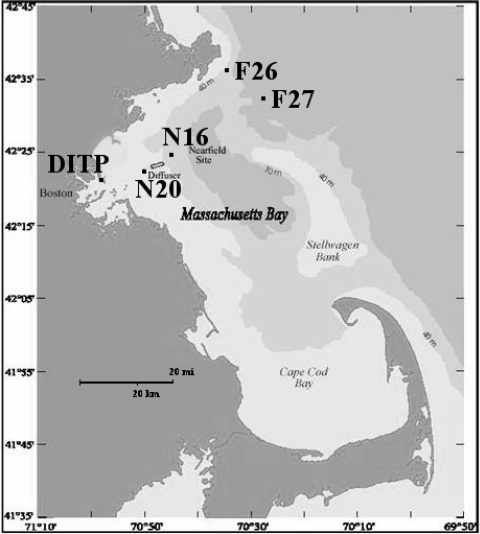
After extensive discussions with Massachusetts Water Resource Authority (MWRA) personnel closely associated with the monitoring program for Massachusetts Bay, three pairs of sampling sites were chosen along the wastewater stream (Fig. 1). The first two consisted of influent and effluent from the DITP. The second pair was selected from among those in the so-called nearfield, the area immediately surrounding the outfall site in Massachusetts Bay. These two sites were chosen in particular because they lie consistently within the densest regions of the plume and tend to have higher fecal coliform counts (54-57). They are identified as N16 (latitude 42°23.64′N, longitude 70°45.18′W) and N20 (latitude 42°22.92′N, longitude 70°49.02′W) (39). The third pair consisted of two sites in the farfield, areas of Massachusetts Bay distant from the outfall site but monitored for potential ecological effects. These two sites were chosen in particular because they have a relatively short residence time and are not measurably affected by the plume, i.e., have effluent dilution greater than 1,000 and ammonium concentrations at background levels (less than 4 μM), as determined by modeling and sampling data, respectively (44). They are identified as F26 (latitude 42°36.12′N, longitude 70°33.90′W) and F27 (latitude 42°33.00′N, longitude 70°26.82′W) (39).
FIG. 1.
Location of DITP and the four ocean sampling sites, two in the nearfield (N16 and N20) and two in the farfield (F26 and F27). Adapted with permission from reference 31.
Water samples were collected from all six sites within a 48-hour period. Thirty-five samples were taken from each site for a total of 210 samples. Sewage influent was sampled first, followed by sewage effluent. Both were collected in 50-ml sterile, narrow-mouth polyethylene bottles. Next, 500-ml seawater samples were collected, first from the nearfield and then from the farfield. Using a Niskin bottle deployed from a boat, seawater was collected from five points in the water column (seven samples from each depth). The previously described method for determining sampling depths at each location is based on the depth of subsurface chlorophyll maximum. This method ensured sampling above, around, and below the existence of a pycnocline and collection of effluent that may be trapped beneath a thermal layer, as Massachusetts Bay is known to be stratified during the summer months, with one turnover event typically occurring in the fall (43, 53). However, since measures of chlorophyll fluorescence were consistent throughout the water column, samples were taken from equally spaced depths, including one near-surface sample and one near-bottom sample. Additionally, physiochemical parameters, including salinity, dissolved oxygen content, temperature, and pH, were measured at all sampling sites and at all depths using a Hydrolab (Loveland, CO) multiparameter probe. All samples were filtered immediately upon collection using 0.2-μm Supor filters and negative pressure. Filters were kept frozen, first in liquid nitrogen and then in a −80°C freezer.
Total nucleic acids were extracted directly from whole filters according to the methods of Kerkhof et al. (35). Briefly, lysis was induced by rapid freeze-thaw cycling and addition of lysozyme, preceded by phenol-chloroform DNA extraction (five samples were lost at random during processing). DNA concentrations were measured using a NanoDrop ND-100 spectrophotometer (NanoDrop, Wilmington, DE).
Development of a universal blaTEM primer/probe set.
The nucleotide sequences for the 135 blaTEM genes described to date were retrieved, either from the GenBank database or directly from the literature, and then aligned using the ClustalW program (76) (Table 1; Fig. 2). The blaTEM genes are very conserved through much of the sequence. The pairwise alignment scores ranged from 88 to 99, and the overall nucleotide identity was 56%, which indicated the potential for a universal primer/probe set (58, 80).
TABLE 1.
TEM β-lactamase gene sequences used for primer and probe designa
| blaTEM | Alternate name(s) | GenBank accession no. | Reference(s) |
|---|---|---|---|
| TEM-1 | RTEM-1 | V00613 | |
| TEM-2 | X54606 | ||
| TEM-3 | CTX-1, TEM-14 | X64523 | |
| TEM-4 | 62, 68 | ||
| TEM-5 | CAZ-1 | 64, 68 | |
| TEM-6 | X57972 | ||
| TEM-7 | AF527798 | ||
| TEM-8 | CAZ-2 | X65252 | |
| TEM-9 | RHH-1 | 5, 70 | |
| TEM-10 | MGH-1, TEM-E3, TEM-23 | U09188 | |
| TEM-11 | CAZ-lo | AY874537 | |
| TEM-12 | YOU-2, CAZ-3, TEM-E2 | M88143 | |
| TEM-13 | 25, 46 | ||
| TEM-15 | 25, 46 | ||
| TEM-16 | CAZ-7 | X65254 | |
| TEM-17 | Y14574 | ||
| TEM-18 | 46 | ||
| TEM-19 | 46 | ||
| TEM-20 | Y17581 | ||
| TEM-21 | Y17582 | ||
| TEM-22 | Y17583 | ||
| TEM-24 | CAZ-6 | X65253 | |
| TEM-25 | CTX-2 | 14, 65 | |
| TEM-26 | YOU-1 | L19940 | |
| TEM-27 | 51 | ||
| TEM-28 | U37195 | ||
| TEM-29 | Y17584 | ||
| TEM-30 | IRT-2, TRI-2, E-GUER | 6, 78 | |
| TEM-31 | IRT-1, TRI-1, E-SAL | 6, 78 | |
| TEM-32 | IRT-3 | 9 | |
| TEM-33 | IRT-5 | 25, 84 | |
| TEM-34 | IRT-6 | 84 | |
| TEM-35 | IRT-4 | 84 | |
| TEM-36 | IRT-7 | 84 | |
| TEM-37 | 27 | ||
| TEM-38 | IRT-9 | 27 | |
| TEM-39 | IRT-10 | 27 | |
| TEM-40 | IRT-167, IRT-11 | 9, 71 | |
| TEM-42 | X98047 | ||
| TEM-43 | U95363 | ||
| TEM-44 | IRT2-2, IT-13 | 13 | |
| TEM-45 | IRT-14 | X95401 | |
| TEM-46 | CAZ-9 | 15 | |
| TEM-47 | Y10279 | ||
| TEM-48 | Y10280 | ||
| TEM-49 | Y10281 | ||
| TEM-50 | CMT-1 | 66 | |
| TEM-51 | IRT-15 | 12 | |
| TEM-52 | Y13612 | ||
| TEM-53 | AF104441 | ||
| TEM-54 | AF104442 | ||
| TEM-55 | 37 | ||
| TEM-56 | 59 | ||
| TEM-57 | 10 | ||
| TEM-58 | 69 | ||
| TEM-59 | IRT-17 | AF062386 | |
| TEM-60 | AF047171 | ||
| TEM-61 | CAZ-hi | 79 | |
| TEM-63 | TEM-64 | AF332513 | |
| TEM-65 | IRT-16 | 10 | |
| TEM-66 | 10 | ||
| TEM-67 | AF091113 | ||
| TEM-68 | AJ239002 | ||
| TEM-70 | AF188199 | ||
| TEM-71 | AF203816 | ||
| TEM-72 | AF157413 | ||
| TEM-73 | IRT-18 | AJ012256 | |
| TEM-74 | IRT-19 | 10 | |
| TEM-75 | AY130284 | ||
| TEM-76 | IRT-20 | AF190694 | |
| TEM-77 | IRT-21 | AF190695 | |
| TEM-78 | IRT-22 | AF190693 | |
| TEM-79 | AF190692 | ||
| TEM-80 | IRT-24 | AF347054 | |
| TEM-81 | AF427127 | ||
| TEM-82 | AF427128 | ||
| TEM-83 | AF427129 | ||
| TEM-84 | AF427130 | ||
| TEM-85 | AJ277414 | ||
| TEM-86 | AJ277415 | ||
| TEM-87 | AF250872 | ||
| TEM-88 | AY027590 | ||
| TEM-89 | AY039040 | ||
| TEM-90 | TLE-1 | AF351241 | |
| TEM-91 | AB049569 | ||
| TEM-92 | 19 | ||
| TEM-93 | AJ318093 | ||
| TEM-94 | AJ318094 | ||
| TEM-95 | AJ308558 | ||
| TEM-96 | AY092401 | ||
| TEM-97 | AF397066 | ||
| TEM-98 | AF397067 | ||
| TEM-99 | AF397068 | ||
| TEM-101 | AF495873 | ||
| TEM-102 | AY029354 | ||
| TEM-103 | IRT-28 | 2 | |
| TEM-104 | AF516719 | ||
| TEM-105 | AF516720 | ||
| TEM-106 | AY101578 | ||
| TEM-107 | AY101764 | ||
| TEM-108 | AF506748 | ||
| TEM-109 | CMT-5 | AY628175 | |
| TEM-110 | AY130283 | ||
| TEM-111 | AF468003 | ||
| TEM-112 | AY589493 | ||
| TEM-113 | AY589494 | ||
| TEM-114 | AY589495 | ||
| TEM-115 | AF535127 | ||
| TEM-116 | AY425988 | ||
| TEM-117 | AY130282 | ||
| TEM-118 | AY130285 | ||
| TEM-120 | AY243512 | ||
| TEM-121 | AY307374 | ||
| TEM-122 | AY307100 | ||
| TEM-123 | AY327539 | ||
| TEM-124 | AY327540 | ||
| TEM-125 | AY628176 | ||
| TEM-126 | AY628199 | ||
| TEM-127 | AY368236 | ||
| TEM-128 | AY368237 | ||
| TEM-129 | AJ746225 | ||
| TEM-130 | AJ866988 | ||
| TEM-131 | AY436361 | ||
| TEM-132 | AY491682 | ||
| TEM-133 | AY528425 | ||
| TEM-134 | AY574271 | ||
| TEM-135 | AJ634602 | ||
| TEM-136 | AY826417 | ||
| TEM-137 | AM286274 | ||
| TEM-138 | AY853593 | ||
| TEM-141 | AY956335 | ||
| TEM-143 | DQ075245 | ||
| TEM-144 | DQ256080 | ||
| TEM-145 | DQ105528 |
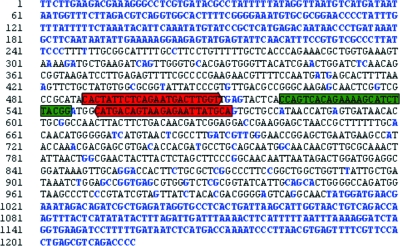
FIG. 2.
Primers and 5′-nuclease assay probe resulting from the alignment of the 135 blaTEM genes. Base pairs that are not conserved are in blue, primers are highlighted in red, and the probe is highlighted in green.
The software program Primer Express 2.0 (Applied Biosystems, Foster City, CA) was used to design a forward primer, a reverse primer, and a probe that would anneal to regions of sequence conserved among all 135 variants of the gene. The primers and probe were optimized, subject to the following constraints. Primer melting temperature was set between 51 and 60°C (optimally 59°C), and the maximum allowable difference between forward and reverse primers was 2°C. Primer GC content was set between 30 and 80%. Primer length was set between 18 and 25 bp (optimally 20 bp). The resulting amplicon was specified to have a melting temperature between 0 and 85°C and a length between 65 and 90 bp. The probe was required to have a melting temperature 10.0°C greater than the melting temperature of the primers and could not begin with G. To ensure specificity for blaTEM genes, it was confirmed that the primers and probe would not anneal to other previously characterized sequences by running a comparison with the GenBank database using the Basic Local Alignment Search Tool (BLAST) program (version 2.2.5) (3, 7). blaTEM primers and probes were synthesized by Biosource Int. (Camarillo, CA). Additionally, the BACT2 primer set, developed by Suzuki et al. (73) to be universal to bacterial 16S rRNA genes, was synthesized by Biosearch Technologies (Novato, CA). Both probes carried a 5′-6-carboxyfluorescein label and a 3′-6-carboxytetramethylrhodamine quencher.
Quantitative PCR.
Duplicate real-time PCRs were run with the ABI Prism 7700 sequence detection system (Applied Biosystems; Foster City, CA) and Qiagen Universal PCR master mix (Valencia, CA) in 25-μl reaction mixtures and under reaction conditions of 50°C for 2 min for the uracil-DNA glycosylase step, 95°C for 15 min, and 45 two-step cycles consisting of 94°C for 15 s and 60°C for 1 min. blaTEM primer and probe concentrations were optimized. The final reaction mixtures for amplifying blaTEM contained 400 nM of both forward and reverse primers and 200 nM of TaqMan probe, while those for amplifying 16S rRNA genes contained 1,500 nM of forward primer, 1,000 nM of reverse primer, and 500 nM of Taqman probe (73). Aside from a pair of no-template controls in each run, 1.5 μl of DNA (corresponding to 7.5 ml of original sample) was added to each reaction mixture.
Gene copy numbers were calculated by amplifying eight serial dilutions of a standard in parallel with the samples. Cloned target genes were used rather than genomic DNA so that copy number could be more easily determined. The blaTEM standard pBR322 (New England Biolabs, Ipswich, MA), which contains TEM-1, was diluted such that blaTEM copy numbers ranged from 1.67 × 103 to 1.71 × 106 per reaction mixture. The 16S rRNA standard EBAC31A08, cloned into Escherichia coli, was kindly provided by M. Suzuki, Chesapeake Biological Laboratory, University of Maryland Center for Environmental Science. After purification using a QIAprep Spin plasmid kit (Qiagen, Valencia, CA) the plasmids were diluted such that the 16S rRNA copy numbers ranged from 7.70 × 100 to 7.70 × 107 per reaction mixture.
To screen for PCR inhibition, standard dilutions were spiked with environmental DNA and the experimental difference was compared to the actual copies of blaTEM genes in the standards. Additionally, blaTEM was amplified in a dilution series of selected samples. Inhibition of the PCR by environmental DNA was not detected.
Data analysis.
All quantitative PCR results were analyzed with a PowerMac 4400 computer (Apple, Cupertino, CA) and Sequence Detector v1.6.3 software (Applied Biosystems, Foster City, CA). Gene copy numbers of replicate pairs were averaged, while ensuring that the coefficient of variation (CV) between replicates was less than 1.
For each of the three outcome analyses, a two-sample t test (independent samples) was performed for each of the six possible combinations of the four general sample types (DITP influent, DITP effluent, nearfield, farfield). Note that the two-sample t test is an approximate nonparametric test statistic for large samples. The resulting P values were examined using the Hochberg step-up procedure to control the overall family-wise error rate (probability of one or more false positives) when simultaneously considering the multiple independent null hypotheses (28, 29). The significance of variations between sublocations in the nearfield and farfield were also analyzed with two-sample t tests (independent samples). To test the null hypothesis that the concentrations of blaTEM genes at all five sampling depths for each particular ocean sampling location were equal, the Kruskal-Wallis test was used. All tests were conducted at the α = 0.05 overall significance level.
RESULTS AND DISCUSSION
Results indicate that the blaTEM Taqman PCR assay is valid and well-suited for quantitative measurements. PCR products were of the expected size, and standard curves, using known copy numbers of the blaTEM standard, were repeatable and consistently provided significant correlations (r2 always >0.99) (data not shown). The observed similarity of slopes between amplification curves indicated constant amplification efficiency. Additionally, there was a relatively high level of analytical precision. In general, replicate measurements had a CV less than 0.15. The average CV for blaTEM copy number was 0.10 (n = 205).
Using measurements of total DNA concentration and 16S rRNA genes, it was possible to estimate how biomass and bacteria, respectively, and the ratio between the two vary between sampling locations (Table 2). As is becoming increasingly common in the literature, measures of 16S rRNA genes were used as a proxy for bacterial count. Deviations from actual bacterial count, particularly overestimations, can result from variability in the number of ribosomal operons, as well as the various biases in the PCR methodology (1, 72). Previous experiments found the genomes of coastal marine isolates to contain between four and nine copies of the 16S rRNA gene (36). Therefore, reported estimates of biomass and bacteria should be used for comparative purposes, and translating 16S gene count directly to bacterial count could lead to overestimates by as much as a factor of 10.
TABLE 2.
Concentration of biomass (measured by total DNA concentration), bacteria (measured by 16S rRNA gene count), and the ratio of the two at each sampling locationa
| Site | DNAT (ng)/ml of sample | Copies of 16S rRNA genes/ml of sample | Copies of 16S rRNA genes/ng of DNAT |
|---|---|---|---|
| Influent | 4,750.9 ± 1,323.7 | 9.5 × 1010 ± 3.8 × 1010 | 2.2 × 107 ± 9.7 × 106 |
| Effluent | 1,714.6 ± 497.9 | 8.3 × 109 ± 1.2 × 1010 | 4.9 × 106 ± 7.0 × 106 |
| Nearfield | 46.2 ± 28.4 | 5.6 × 107 ± 3.5 × 107 | 1.6 × 106 ± 1.7 × 106 |
| N16 | 36.1 ± 18.6 | 3.5 × 107 ± 2.0 × 107 | 1.3 × 106 ± 9.3 × 105 |
| N20 | 56.4 ± 32.8 | 7.8 × 107 ± 3.5 × 107 | 2.0 × 106 ± 2.2 × 106 |
| Farfield | 35.7 ± 23.1 | 8.5 × 107 ± 4.8 × 107 | 3.0 × 106 ± 2.1 × 106 |
| F26 | 43.3 ± 27.4 | 9.0 × 107 ± 4.6 × 107 | 2.8 × 106 ± 2.1 × 106 |
| F27 | 27.7 ± 13.9 | 7.9 × 107 ± 5.1 × 107 | 3.1 × 106 ± 2.2 × 106 |
All values are means ± standard deviations. rRNA gene counts were determined by quantitative PCR.
As expected, sewage treatment leads to a significant reduction in biomass and an approximate 1-order of magnitude drop in bacteria. Since sewage treatment processes, such as chlorination, specifically target bacteria it was not surprising to see a drop in the ratio of bacteria to biomass with treatment. Because these conclusions are based on measurements of DNA and not whole cells, the results suggest that the treatment process not only destroys intact cells but removes the remaining DNA, perhaps by nuclease degradation, absorption onto sludge, or a combination of the two (18). Levels of both biomass and bacteria are approximately 2 orders of magnitude higher in the sewage effluent compared to the bay into which it flows. Among the sampling sites in the bay, concentrations of biomass and bacteria are similar.
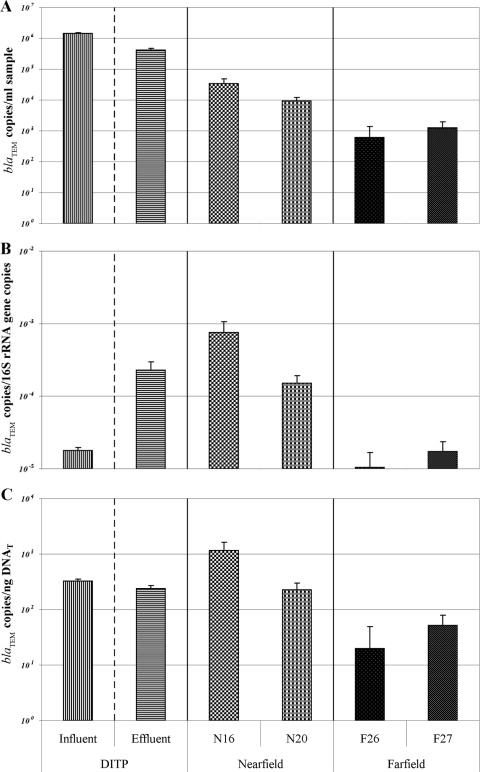
By applying the blaTEM Taqman assay, it was found that while modern sewage treatment technologies reduce the concentrations of these antibiotic resistance genes, they are being introduced into the environment through sewage effluent in much higher concentrations than occur naturally, thus creating reservoirs of increased resistance potential (Fig. 3A). More specifically, the concentration of blaTEM genes in untreated sewage is 1.4 ×106, while treatment causes the concentration to drop to 4.1 × 105 in the effluent. Once it is mixed with seawater in the nearfield, the concentration drops again to 2.2 × 104. This is compared to an area of Massachusetts Bay unaffected by the sewage outfall, which has a concentration of 9.2 × 102 blaTEM genes. While controlling the overall false-positive rate (family-wise error rate), all of these differences easily meet the criteria set for statistical significance at 0.05 (Table 3), since the overall significance level is 4.93 × 10−3.
FIG. 3.
blaTEM copy number per ml of sample (A), relative to bacterial count (as estimated by 16S rRNA copy number) (B), and relative to biomass (as estimated by total DNA concentration) (C). Gene copy numbers were determined by quantitative PCR. Bars represent means. Error bars represent standard errors.
TABLE 3.
P values from comparisons of blaTEM copy numbers between sites
| Site comparison |
P value from comparison of blaTEM copy no. pera:
|
||
|---|---|---|---|
| ml of sample | 16S rRNA gene copy | ng of DNAT | |
| Influent vs effluent | 1.49 × 10−14* | 3.81 × 10−3* | 4.38 × 10−2 |
| Influent vs nearfield | 3.96 × 10−31* | 8.27 × 10−3* | 1.35 × 10−1 |
| Influent vs farfield | 2.49 × 10−31* | 2.90 × 10−1 | 5.09 × 10−16* |
| Effluent vs nearfield | 3.72 × 10−9* | 2.06 × 10−1 | 6.55 × 10−2 |
| Effluent vs farfield | 6.51 × 10−10* | 2.90 × 10−3* | 1.45 × 10−7* |
| Nearfield vs farfield | 4.93 × 10−3* | 7.45 × 10−3* | 7.59 × 10−3* |
Based on two-sample t tests used in conjunction with the Hochberg step-up procedure (overall α = 0.05). *, statistically significant.
Again using copies of 16S rRNA genes as a proxy for bacterial count, normalizing the concentrations of blaTEM genes to this measure describes how levels of β-lactamase encoding material vary with bacterial count. This ratio is dramatically higher in sewage effluent (2.3 × 10−4) compared to sewage influent (1.8 × 10−5), suggesting that while the treatment process removes bacteria, antibiotic-resistant bacteria are more likely to survive the treatment process (Fig. 3B). Similarly, another study that measured the percentage of coliforms resistant to ampicillin also reported an increase in treated wastewater compared to untreated (32). Results by Morozzi (52) suggest that the addition of chlorine in the final stages of sewage treatment may coselect for chlorine- and antibiotic-tolerant strains. The increased survival ability during sewage treatment processes by antibiotic-resistant bacteria seems, in the case of Massachusetts Bay, to lead to the nearfield containing bacterial DNA with higher proportions of β-lactamase genes (4.5 × 10−4) compared to the farfield (1.4 × 10−5). Thus, while the total concentration of the bacteria in the bay is largely unaffected by the plume, the bacteria in the vicinity of the outfall appear more likely to harbor β-lactamase genes. The ratio of blaTEM genes to 16S rRNA genes is statistically significantly different between the influent and the effluent, the influent and nearfield, the effluent and the farfield, and the nearfield and the farfield (Table 3).
By using the ratio of blaTEM genes to total DNA concentration, it was possible to compare the concentration of β-lactamase genes relative to biomass among the sampling locations (Fig. 3C). Since the concentrations of total DNA are approximately 2 orders of magnitude higher in the sewage compared to the seawater, when normalizing on this measure, the levels of blaTEM between these two general sample types tend to level out. With this normalization, there are still statistically significant higher concentrations of β-lactamase genes in each of the influent (3.2 × 102), the effluent (2.4 × 102), and the nearfield (6.9 × 102) samples/area, compared to the farfield (3.5 × 101) (Table 3).
The data do not reveal any striking differences between subsites. There is no statistically significant difference either between N16 and N20 or between F26 and F27 for any of the three outcome measures (blaTEM copies/ml of sample, blaTEM copies/16S rRNA gene copies, and blaTEM copies/ng of total DNA [DNAT]). Visual inspection of the data does not indicate any clear trends with regard to sampling depth in the bay. Also, Kruskal-Wallis tests reveal no statistically significant difference in the number of blaTEM genes between the five sampling depths at each of four ocean sampling sites. The physiochemical hydrological parameters, such as temperature, also show little variation with depth (data not shown). These data, along with the fact that the samples were collected in late Fall, indicate that by the time the sampling trip occurred, the thermocline in Massachusetts Bay had disappeared and the water was well mixed.
Most previous studies regarding resistance to antibiotics, such as β-lactams, and sewage-contaminated waters have been based on phenotypic analysis, and many of these also reported an association (17, 24, 32, 45, 50). Two major drawbacks of assessing resistance by phenotype are that it relies on culturing and that it does not provide information on the mechanism of resistance. For example, characterizing the phenotype would not distinguish between β-lactam resistance mediated by β-lactamases and that mediated by penicillin binding proteins. These two mechanisms have substantially different clinical treatment implications (77). Environmental antibiotic resistance genotyping studies have predominately focused on tetracycline resistance in animal waste lagoons (16, 23, 63, 67, 83). However, plasmids encoding β-lactam resistance have previously been isolated from human wastewater and have been shown to be transferable both in vitro and in situ (20, 47, 74, 75). It is important to note that results from genotype-based studies that analyze DNA, such as this one, do not provide information regarding the expression of these antibiotic resistance genes. However, expression is not the central question when, as here, the goal is an evaluation of the degree to which ecosystems serve as pools of resistance genes that could potentially be transferred to pathogens.
Acknowledgments
We thank Andrea Rex, Michael Mickelson, and Kenneth Keay at the MWRA for providing us with MWRA water quality monitoring data and helping to identify sampling sites and Steve Rhode, Edward Caruso, and Cara Seaman, also of the MWRA, for facilitating the collection of sewage. We also thank M. Suzuki for providing strain EBAC31A08.
This publication was made possible by grant number 5 P42 ES05947 from the National Institute of Environmental Health Sciences, NIH, which we gratefully acknowledge.
This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Published ahead of print on 7 November 2008.
REFERENCES
- 1.Acinas, S. G., L. A. Marcelino, V. Klepac-Ceraj, and M. F. Polz. 2004. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J. Bacteriol. 186:2629-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, R., G. Gerbaud, M. Galimand, and P. Courvalin. 2002. TEM-103/IRT-28 β-lactamase, a new TEM variant produced by Escherichia coli BM4511. Antimicrob. Agents Chemother. 46:3627-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber, M., and J. E. M. Whitehead. 1949. Bacteriophage types in penicillin-resistant staphylococcal infections. Br. Med. J. 2:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauernfeind, A., and G. Horl. 1987. Novel R-factor borne β-lactamase of Escherichia coli conferring resistance to cephalosporins. Infection 15:257-259. [DOI] [PubMed] [Google Scholar]
- 6.Belaaouaj, A., C. Lapoumeroulie, M. M. Canica, G. Vedel, P. Nevot, R. Krishnamoorthy, and G. Paul. 1994. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2). FEMS Microbiol. Lett. 120:75-80. [DOI] [PubMed] [Google Scholar]
- 7.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, B. A. Rapp, and D. L. Wheeler. 2000. GenBank. Nucleic Acids Res. 28:15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand, X., M. Thouverez, P. Bailly, C. Cornette, D. Talon, et al. 2000. Clinical and molecular epidemiology of hospital Enterococcus faecium isolates in eastern France. J. Hosp. Infect. 45:125-134. [DOI] [PubMed] [Google Scholar]
- 9.Blazquez, J., M. R. Baquero, R. Canton, I. Alos, and F. Baquero. 1993. Characterization of a new TEM-type β-lactamase resistant to clavulanate, sulbactam, and tazobactam in a clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 37:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnet, R., C. De Champs, D. Sirot, C. Chanal, R. Labia, and J. Sirot. 1999. Diversity of TEM mutants in Proteus mirabilis. Antimicrob. Agents Chemother. 43:2671-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bret, L., E. B. Chaibi, C. Chanal-Claris, D. Sirot, R. Labia, and J. Sirot. 1997. Inhibitor-resistant TEM (IRT) β-lactamases with different substitutions at position 244. Antimicrob. Agents Chemother. 41:2547-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bret, L., C. Chanal, D. Sirot, R. Labia, and J. Sirot. 1996. Characterization of an inhibitor-resistant enzyme IRT-2 derived from TEM-2 β-lactamase produced by Proteus mirabilis strains. J. Antimicrob. Chemother. 38:183-191. [DOI] [PubMed] [Google Scholar]
- 14.Chanal, C., D. Sirot, H. Malaure, M. C. Poupart, and J. Sirot. 1994. Sequences of CAZ-3 and CTX-2 extended-spectrum β-lactamase genes. Antimicrob. Agents Chemother. 38:2452-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanal-Claris, C., D. Sirot, L. Bret, P. Chatron, R. Labia, and J. Sirot. 1997. Novel extended-spectrum TEM-type β-lactamase from an Escherichia coli isolate resistant to ceftazidime and susceptible to cephalothin. Antimicrob. Agents Chemother. 41:715-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chee-Sanford, J. C., R. I. Aminov, I. J. Krapac, N. Garrigues-Jeanjean, and R. I. Mackie. 2001. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 67:1494-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke, M. D. 1976. Antibiotic resistance among coliform and fecal coliform bacteria isolated from sewage, seawater, and marine shellfish. Antimicrob. Agents Chemother. 9:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davison, J. 1999. Genetic exchange between bacteria in the environment. Plasmid 42:73-91. [DOI] [PubMed] [Google Scholar]
- 19.De Champs, C., C. Monne, R. Bonnet, W. Sougakoff, D. Sirot, C. Chanal, and J. Sirot. 2001. New TEM variant (TEM-92) produced by Proteus mirabilis and Providencia stuartii isolates. Antimicrob. Agents Chemother. 45:1278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Droge, M., A. Puhler, and W. Selbitschka. 2000. Phenotypic and molecular characterization of conjugative antibiotic resistance plasmids isolated from bacterial communities of activated sludge. Mol. Gen. Genet. 263:471-482. [DOI] [PubMed] [Google Scholar]
- 21.Elwell, L. P., J. De Graaff, D. Seibert, and S. Falkow. 1975. Plasmid-linked ampicillin resistance in Haempohilus influenza type b. Infect. Immun. 12:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elwell, L. P., M. Roberts, L. W. Mayer, and S. Falkow. 1977. Plasmid-mediated β-lactamase production in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 11:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engemann, C. A., L. Adams, C. W. Knapp, and D. W. Graham. 2006. Disappearance of oxytetracycline resistance genes in aquatic systems. FEMS Microbiol. Lett. 263:176-182. [DOI] [PubMed] [Google Scholar]
- 24.Goni-Urriza, M., M. Capdepuy, C. Arpin, N. Raymond, P. Caumette, and C. Quentin. 2000. Impact of an urban effluent on antibiotic resistance of riverine Enterobacteriaceae and Aeromonas spp. Appl. Environ. Microbiol. 66:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goussard, S., and P. Courvalin. 1999. Updated sequence information for TEM β-lactamase genes. Antimicrob. Agents Chemother. 43:367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall, B. G., and M. Barlow. 2004. Evolution of the serine β-lactamases: past, present and future. Drug Resist. Update 7:111-123. [DOI] [PubMed] [Google Scholar]
- 27.Henquell, C., C. Chanal, D. Sirot, R. Labia, and J. Sirot. 1995. Molecular characterization of nine different types of mutants among 107 inhibitor-resistant TEM β-lactamases from clinical isolates of Escherichia coli. Antimicrob. Agents Chemother. 39:427-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochberg, Y. 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800-802. [Google Scholar]
- 29.Hochberg, Y., and Y. Benjamini. 1990. More powerful procedures for multiple significance testing. Stat. Med. 9:811-818. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh, S. R. 2000. Antimicrobial susceptibility and species identification for clinical isolates of enterococci. J. Microbiol. Immunol. Infect. 33:253-257. [PubMed] [Google Scholar]
- 31.Hunt, C., A. Mansfield, P. Roberts, C. Albro, W. Geyer, W. Steinhauer, and M. Mickelson. 2002. Massachusetts Water Resources Authority outfall effluent dilution: July 2001 report, ENQUAD 2002-07. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/enquad/pdf/2002-07.pdf
- 32.Iwane, T., T. Urase, and K. Yamamoto. 2001. Possible impact of treated wastewater discharge on incidence of antibiotic resistant bacteria in river water. Water Sci. Technol. 43:91-99. [PubMed] [Google Scholar]
- 33.Jacoby, G., and K. Bush. 2006, posting date. Amino acid sequences for TEM, SHV and OXA extended-spectrum and inhibitor resistant β-lactamases. www.lahey.org/studies/webt.asp.
- 34.Jacoby, G. A., and K. Bush. 2005. β-Lactam resistance in the 21st century, p. 570. In D. G. White, M. N. Alekshun, and P. F. McDermott (ed.), Frontiers in antimicrobial resistance: a tribute to Stuart B. Levy. ASM Press, Washington, DC.
- 35.Kerkhof, L., M. Santoro, and J. Garland. 2000. Response of soybean rhizosphere communities to human hygiene water addition as determined by community level physiological profiling (CLPP) and terminal restriction fragment length polymorphism (TRFLP) analysis. FEMS Microbiol. Lett. 184:95-101. [DOI] [PubMed] [Google Scholar]
- 36.Kerkhof, L., and M. Speck. 1997. Ribosomal RNA gene dosage in marine bacteria. Mol. Mar. Biol. Biotechnol. 6:264-271. [PubMed] [Google Scholar]
- 37.Labia, R., A. Morand, E. B. Chaibi, P. J., M. Barthelemy, X. Cavallo, F. Grosbost, and D. Sirot. 1997. A clavulanate-susceptible TEM-derived β-lactamase of isoelectric point 5.2 with altered kinetic constants, abstr. C-189. Progr. Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Canada.
- 38.Lachmayr, K. 2007. Anthropogenically induced reservoirs of antibiotic resistance: the case of Massachusetts Bay. Harvard University, Boston, MA.
- 39.Leo, W., W. Andruchow, M. F. Delaney, and L. Wong. 2004. Combined work/quality assurance project plan (CW/QAPP) for nutrient and chlorophyll analysis for outfall monitoring, ms-089. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/enquad/pdf/ms-089.pdf.
- 40.Levesque, R. C., and G. A. Jacoby. 1988. Molecular structure and interrelationships of multiresistance β-lactamase transposons. Plasmid 19:21-29. [DOI] [PubMed] [Google Scholar]
- 41.Levy, S. B. 2002. Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 49:25-30. [DOI] [PubMed] [Google Scholar]
- 42.Levy, S. B., G. B. FitzGerald, and A. B. Macone. 1976. Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm. N. Engl. J. Med. 295:583-588. [DOI] [PubMed] [Google Scholar]
- 43.Libby, S., C. Gagnon, C. Albro, M. Mickelson, A. Keller, D. Borkman, J. Turner, and C. Oviatt. 2002. Combined work/quality assurance project plan (CWQAPP) for water quality monitoring: 2002-2005, ms-074. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/enquad/pdf/ms-074.pdf.
- 44.Libby, S., R. Geyer, A. Keller, J. Turner, D. Borkman, C. Oviatt, and C. Hunt. 2003. 2002 annual water column monitoring report, ENQUAD 2003-09. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/enquad/pdf/2003-09.pdf.
- 45.Linton, K. B., M. H. Richmond, R. Bevan, and W. A. Gillespie. 1974. Antibiotic resistance and R factors in coliform bacilli isolated from hospital and domestic sewage. J. Med. Microbiol. 7:91-103. [DOI] [PubMed] [Google Scholar]
- 46.Mabilat, C., and P. Courvalin. 1990. Development of “oligotyping” for characterization and molecular epidemiology of TEM β-lactamases in members of the family Enterobacteriaceae. Antimicrob. Agents Chemother. 34:2210-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mach, P. A., and D. J. Grimes. 1982. R-plasmid transfer in a wastewater treatment plant. Appl. Environ. Microbiol. 44:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthew, M. 1979. Plasmid-mediated β-lactamases of gram-negative bacteria: properties and distribution. J. Antimicrob. Chemother. 5:349-358. [DOI] [PubMed] [Google Scholar]
- 49.Medeiros, A. A. 1984. β-Lactamases. Br. Med. Bull. 40:18-27. [DOI] [PubMed] [Google Scholar]
- 50.Mesa, R. J., V. Blanc, A. R. Blanch, P. Cortes, J. J. Gonzalez, S. Lavilla, E. Miro, M. Muniesa, M. Saco, M. T. Tortola, B. Mirelis, P. Coll, M. Llagostera, G. Prats, and F. Navarro. 2006. Extended-spectrum β-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58:211-215. [DOI] [PubMed] [Google Scholar]
- 51.Morosini, M. I., R. Canton, J. Martinez-Beltran, M. C. Negri, J. C. Perez-Diaz, F. Baquero, and J. Blazquez. 1995. New extended-spectrum TEM-type β-lactamase from Salmonella enterica subsp. enterica isolated in a nosocomial outbreak. Antimicrob. Agents Chemother. 39:458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morozzi, G., G. Cenci, G. Caldini, R. Sportolari, and A. G. Bahojbi. 1989. Development of antibiotic resistance in purified sewage effluents subjected to chlorination. Ann. Ig. 1:351-362. [In Italian.] [PubMed] [Google Scholar]
- 53.MWRA. 2003. Briefing for OMSAP workshop of ambient monitoring revisions, ENQUAD ms-085. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/enquad/pdf/ms-085.pdf.
- 54.MWRA. 2001. Massachusetts Bay bacteria counts, 2001. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/pdf/2001mb_bacteria.pdf.
- 55.MWRA. 2002. Massachusetts Bay bacteria counts, 2002. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/pdf/2002mb_bacteria.pdf.
- 56.MWRA. 2003. Massachusetts Bay bacteria counts, 2003. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/pdf/2003mb_bacteria.pdf.
- 57.MWRA. 2004. Massachusetts Bay bacteria counts, 2004. Massachusetts Water Resources Authority, Boston, MA. http://www.mwra.state.ma.us/harbor/pdf/2004_mb_bacteria.pdf.
- 58.Myers, E. W., and W. Miller. 1988. Optimal alignments in linear space. Comput. Appl. Biosci. 4:11-17. [DOI] [PubMed] [Google Scholar]
- 59.Neuwirth, C., R. Labia, E. Siebor, A. Pechinot, S. Madec, E. B. Chaibi, and A. Kazmierczak. 2000. Characterization of TEM-56, a novel β-lactamase produced by a Klebsiella pneumoniae clinical isolate. Antimicrob. Agents Chemother. 44:453-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newton, G. G., and E. P. Abraham. 1956. Isolation of cephalosporin C, a penicillin-like antibiotic containing D-alpha-aminoadipic acid. Biochem. J. 62:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parry, C. M. 2003. Antimicrobial drug resistance in Salmonella enterica. Curr. Opin. Infect. Dis. 16:467-472. [DOI] [PubMed] [Google Scholar]
- 62.Paul, G. C., G. Gerbaud, A. Bure, A. M. Philippon, B. Pangon, and P. Courvalin. 1989. TEM-4, a new plasmid-mediated β-lactamase that hydrolyzes broad-spectrum cephalosporins in a clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 33:1958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peak, N., C. W. Knapp, R. K. Yang, M. M. Hanfelt, M. S. Smith, D. S. Aga, and D. W. Graham. 2007. Abundance of six tetracycline resistance genes in wastewater lagoons at cattle feedlots with different antibiotic use strategies. Environ. Microbiol. 9:143-151. [DOI] [PubMed] [Google Scholar]
- 64.Petit, A., D. L. Sirot, C. M. Chanal, J. L. Sirot, R. Labia, G. Gerbaud, and R. A. Cluzel. 1988. Novel plasmid-mediated β-lactamase in clinical isolates of Klebsiella pneumoniae more resistant to ceftazidime than to other broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 32:626-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poupart, M. C., C. Chanal, D. Sirot, R. Labia, and J. Sirot. 1991. Identification of CTX-2, a novel cefotaximase from a Salmonella mbandaka isolate. Antimicrob. Agents Chemother. 35:1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sirot, D., C. Recule, E. B. Chaibi, L. Bret, J. Croize, C. Chanal-Claris, R. Labia, and J. Sirot. 1997. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 41:1322-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith, M. S., R. K. Yang, C. W. Knapp, Y. Niu, N. Peak, M. M. Hanfelt, J. C. Galland, and D. W. Graham. 2004. Quantification of tetracycline resistance genes in feedlot lagoons by real-time PCR. Appl. Environ. Microbiol. 70:7372-7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sougakoff, W., A. Petit, S. Goussard, D. Sirot, A. Bure, and P. Courvalin. 1989. Characterization of the plasmid genes blaT-4 and blaT-5 which encode the broad-spectrum β-lactamases TEM-4 and TEM-5 in Enterobacteriaceae. Gene 78:339-348. [DOI] [PubMed] [Google Scholar]
- 69.Speldooren, V., B. Heym, R. Labia, and M. H. Nicolas-Chanoine. 1998. Discriminatory detection of inhibitor-resistant β-lactamases in Escherichia coli by single-strand conformation polymorphism-PCR. Antimicrob. Agents Chemother. 42:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spencer, R. C., P. F. Wheat, T. G. Winstanley, D. M. Cox, and S. J. Plested. 1987. Novel β-lactamase in a clinical isolate of Klebsiella pneumoniae conferring unusual resistance to β-lactam antibiotics. J. Antimicrob. Chemother. 20:919-921. [DOI] [PubMed] [Google Scholar]
- 71.Stapleton, P., P. J. Wu, A. King, K. Shannon, G. French, and I. Phillips. 1995. Incidence and mechanisms of resistance to the combination of amoxicillin and clavulanic acid in Escherichia coli. Antimicrob. Agents Chemother. 39:2478-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki, M. T., L. T. Taylor, and E. F. DeLong. 2000. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 66:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szczepanowski, R., I. Krahn, B. Linke, A. Goesmann, A. Puhler, and A. Schluter. 2004. Antibiotic multiresistance plasmid pRSB101 isolated from a wastewater treatment plant is related to plasmids residing in phytopathogenic bacteria and carries eight different resistance determinants including a multidrug transport system. Microbiology 150:3613-3630. [DOI] [PubMed] [Google Scholar]
- 75.Tennstedt, T., R. Szczepanowski, S. Braun, A. Puhler, and A. Schluter. 2003. Occurrence of integron-associated resistance gene cassettes located on antibiotic resistance plasmids isolated from a wastewater treatment plant. FEMS Microbiol. Ecol. 45:239-252. [DOI] [PubMed] [Google Scholar]
- 76.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.U.S. Congress, Office of Technology Assessment. 1995. Impacts of antibiotic-resistant bacteria, OTA-H-629. U.S. Government Printing Office, Washington, DC.
- 78.Vedel, G., A. Belaaouaj, L. Gilly, R. Labia, A. Philippon, P. Nevot, and G. Paul. 1992. Clinical isolates of Escherichia coli producing TRI β-lactamases: novel TEM-enzymes conferring resistance to β-lactamase inhibitors. J. Antimicrob. Chemother. 30:449-462. [DOI] [PubMed] [Google Scholar]
- 79.Vuye, A., G. Verschraegen, and G. Claeys. 1989. Plasmid-mediated β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli resistant to ceftazidime. Antimicrob. Agents Chemother. 33:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilbur, W. J., and D. J. Lipman. 1983. Rapid similarity searches of nucleic acid and protein data banks. Proc. Natl. Acad. Sci. USA 80:726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.World Health Organization. 1996. The world health report. Geneva, Switzerland.
- 82.Yang, Y., N. Bhachech, P. A. Bradford, B. D. Jett, D. F. Sahm, and K. Bush. 1998. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli isolates producing TEM-10 and TEM-43 β-lactamases from St. Louis, Missouri. Antimicrob. Agents Chemother. 42:1671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu, Z., F. C. Michel, Jr., G. Hansen, T. Wittum, and M. Morrison. 2005. Development and application of real-time PCR assays for quantification of genes encoding tetracycline resistance. Appl. Environ. Microbiol. 71:6926-6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou, X. Y., F. Bordon, D. Sirot, M. D. Kitzis, and L. Gutmann. 1994. Emergence of clinical isolates of Escherichia coli producing TEM-1 derivatives or an OXA-1 β-lactamase conferring resistance to β-lactamase inhibitors. Antimicrob. Agents Chemother. 38:1085-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]