Abstract
Food-borne Listeria monocytogenes is a serious threat to human health, and new strategies to combat this opportunistic pathogen in foods are needed. Bacteriophages are natural enemies of bacteria and are suitable candidates for the environmentally friendly biocontrol of these pathogens. In a comprehensive set of experiments, we have evaluated the virulent, broad-host-range phages A511 and P100 for control of L. monocytogenes strains Scott A (serovar 4b) and WSLC 1001 (serovar 1/2a) in different ready-to-eat (RTE) foods known to frequently carry the pathogen. Food samples were spiked with bacteria (1 × 103 CFU/g), phage added thereafter (3 × 106 to 3 × 108 PFU/g), and samples stored at 6°C for 6 days. In liquid foods, such as chocolate milk and mozzarella cheese brine, bacterial counts rapidly dropped below the level of direct detection. On solid foods (hot dogs, sliced turkey meat, smoked salmon, seafood, sliced cabbage, and lettuce leaves), phages could reduce bacterial counts by up to 5 log units. Variation of the experimental conditions (extended storage over 13 days or storage at 20°C) yielded similar results. In general, the application of more phage particles (3 × 108 PFU/g) was more effective than lower doses. The added phages retained most of their infectivity during storage in foods of animal origin, whereas plant material caused inactivation by more than 1 log10. In conclusion, our data demonstrate that virulent broad-host-range phages, such as A511 and P100, can be very effective for specific biocontrol of L. monocytogenes in contamination-sensitive RTE foods.
Listeria monocytogenes is an opportunistic human pathogen, widely distributed in the environment and transmitted to humans and animals via contaminated foods (50). The organism is well adapted to very different environmental conditions encountered in foods; it tolerates high levels of salt content (10 to 20%), can grow at pH values below 6, with low oxygen, and at temperatures down to 1°C (48). L. monocytogenes causes listeriosis, a severe disease which may result in septicemia, meningitis, encephalitis, or loss of the fetus during pregnancy (52). Although listeriosis is comparatively rare compared to other food-borne infections, the high mortality rate of 15 to 40% is of great concern (49, 52). Cases of sporadic and epidemic listeriosis are increasing (4, 6, 11), and it has been estimated that about 2,000 hospitalizations and 500 deaths occur annually in the United States as a result of the consumption of Listeria in foods (39). Although many foods can serve as vehicles for this pathogen, Listeria was often isolated from ready-to-eat (RTE) foods, such as milk and cheeses, cold-cut meats, smoked fish, seafood, and vegetables (45). RTE foods have been implicated in most of the major listeriosis outbreaks in the last 30 years (13, 18, 20, 21, 42, 45-47). Of particular concern is the fact that they are consumed directly, without a final bactericidal processing step. Since the preservation methods applicable to minimally processed RTE foods often seem to be insufficient to prevent Listeria contamination and growth, novel approaches are needed.
Bacteriophages represent natural enemies of bacteria; they are extremely specific regarding their bacterial hosts and generally do not cross taxonomic boundaries. With respect to the application of phages to foods, their inherent specificity results in the elimination of only the target organisms without compromising the viability of other, autochthonic bacteria in the habitat. This is a desired property of an antimicrobial agent for use in foods; it helps in maintaining product quality, especially in the case of fermented foods and other products produced with the aid of bacteria. Phages are widely distributed in the environment (12) and represent part of the natural microbiological flora of foods (27, 54, 55). Especially suitable for biocontrol purposes are virulent (strictly lytic) phages; they cannot integrate their genome into the bacterial chromosome to form lysogens and will always lyse and kill infected target cells.
The current standing of the use of phages against undesired bacteria in food systems has been summarized previously (22, 26, 44). Briefly, phages were tested in foods contaminated with strains of Campylobacter (19, 35), Escherichia coli (1, 41), Enterobacter (28), Pseudomonas (17, 23), Brochothrix (24), Salmonella (31, 33, 40, 43, 53), and Listeria (10, 16, 32, 33, 34). However, a weak point of most approaches was the use of uncharacterized, sometimes temperate phages. With the use of a virulent, broad-host-range phage, the elimination of Listeria from artificially contaminated soft cheese was reported (10). In the same study, the authors have also presented data regarding the safety of the phage for human consumption, and phage P100 recently received GRAS status for application to foods (5).
The aim of this study was a comprehensive evaluation of virulent Listeria phages for biocontrol in a range of RTE foods (meat, fish, dairy, and plant). Toward this end, we have used the broad-host-range phage A511 (and, to a lesser extent, P100), which can infect about 95% of L. monocytogenes strains of the major serovar groups 1/2 and 4 (10, 36). These viruses are members of the Myoviridae (10, 56), and because of their virulent nature, inevitably kill the host cell once an infection has been established (56). Important with respect to their application to foods is that they lack the genetic functions required for integration of their genome (10, 29) and cannot transduce bacterial DNA (25). The latter is due to the unusual structure of their genome, featuring long terminal repeats, which prevents accidental packaging of host DNA (29). We found it necessary to use a sufficiently high phage concentration to kill the bacteria by primary infection, without relying on self-amplification. A high density of phage particles also increases the probability that the nonmotile phage particles can actually reach the target cells since they do so only by diffusion. Both A511 and P100 were able to strongly decrease the number of viable Listeria cells, but their efficiency was dependent on several intrinsic and extrinsic parameters, such as phage concentration, food matrix, and storage conditions.
MATERIALS AND METHODS
Bacteria and phages.
Listeria monocytogenes WSLC 1001 (serovar 1/2c) and Scott A (serovar 4b) and Listeria ivanovii WSLC 3009 (serovar 5) were grown in half-concentrated brain-heart infusion medium (BHI 1/2) (Biolife, Milan, Italy) at 30°C for 16 h. For the phage indicator strain L. ivanovii WSLC 3009 Cmr, chloramphenicol (7.5 μg/ml) (Sigma, Buchs, Switzerland) was added to the medium. This strain has been genetically engineered for drug resistance by the integration of plasmid pPL2 (30). Phages A511 (29, 36, 38) and P100 (10) were propagated as previously described, using Listeria ivanovii WSLC 3009 as the host (10, 37). The final concentration of the purified phage suspensions was 3 × 1011 PFU/ml, and they were stored at 4°C until use. The efficiency of plating of the phages on the two Listeria monocytogenes strains tested here was not different (results not shown).
Food samples.
Eight different foods were selected to cover the spectrum of fresh, chilled, RTE foods frequently found to be contaminated with Listeria: hot dogs (sausages), cooked and sliced turkey breast meat (cold cuts), smoked salmon, mixed seafood (cooked and chilled cocktail of shrimp, mussels, and calamari), chocolate milk (pasteurized, 3.5% fat), mozzarella cheese brine (unsalted pasteurized whey from plastic bag containers containing fresh mozzarella cheese), iceberg lettuce (leaves), and cabbage (sliced fresh leaves). All foods were purchased at local groceries and initially screened for contamination with Listeria spp. according to EN ISO 11290 part 1:1997 (7) or IDF standard 143A:1995 (8). If applicable, foods were stored frozen at −80°C until use. Lettuce and cabbage were used fresh.
Contamination procedure.
Overnight cultures of L. monocytogenes were diluted 1:5 in fresh medium, incubated for 2 to 3 h at 30°C until an optical density at 600 nm of approximately 0.4 was reached, and decimally diluted in phosphate-buffered saline (100 mM NaCl, 20 mM Na2HPO4, pH 7.4) to the desired cell numbers. The target viable count in spiked foods was 103 CFU/g, and the inoculum volumes were approximately 1% of the total sample size. For experiments lasting 6 days, 60 ± 2 (mean ± standard deviation) g was used, and for experiments lasting 13 days, 100 ± 3 g was used. Before the addition of phage, spiked food samples were incubated at 6°C for 1 to 2 h, allowing the bacteria to adapt to the environmental conditions.
Phage treatment.
To the food samples receiving phage, aliquots of A511 (0.5 to 1.0 ml) were added to achieve a target concentration of 3 × 108 PFU/g or ml. Samples were then incubated at 6°C (to simulate refrigerator storage temperature) for a total of 6 days, unless otherwise noted.
In order to investigate the effects of the different variables and parameters on the efficacy of phage challenge, additional experiments were performed (indicated below and in the figure legends) with lower phage concentrations (3 × 106 and 3 × 107 PFU/g), longer incubation periods (up to 13 days), a higher incubation temperature (20°C), and using phage P100 instead of A511.
Monitoring bacterial and phage counts.
The bacterial viable counts (CFU/g) and phage concentrations (PFU/g) were initially determined immediately after the respective addition of bacteria and phage and monitored at 6 h and 1, 2, 3, and 6 days. For this purpose, 10-g amounts of solid foods were homogenized in 90 ml citrate homogenization buffer by using a stomacher lab blender for 2 to 3 min. For quantitative determination of Listeria cell counts, larger aliquots (1 ml) of the homogenates or the liquid test samples were directly surface plated on 145-mm Oxford agar plates (Oxoid, Cambridge, United Kingdom) or small aliquots (0.1 ml) of the decimal dilutions on 90-mm plates. The plates were incubated for 48 h at 37°C until typical Listeria colonies could be enumerated. The relevant lower detection limits were 1 CFU/ml for liquids (direct plating possible) and 10 CFU/g for solid foods (homogenates represented 10−1 dilutions).
Infective phage remaining in the foods were enumerated as described earlier (10), employing drug-resistant L. ivanovii (WSLC 3009 Cmr) as the phage indicator strain in order to enable direct plating and to prevent contamination of the plates by background flora. Aliquots of 0.1 ml of decimal dilutions from the food samples were mixed with 200 μl host cells and 4 ml molten BHI soft agar (0.4% agar) containing 7.5 μg/ml chloramphenicol. The suspension was poured onto solid agar plates and incubated overnight at 30°C until plaques could be enumerated. We found no evidence that phage infectivity was affected by the homogenization procedure in the stomacher.
When Listeria cell counts at the end of the experiment exceeded 10 CFU/g or 10 CFU/ml in phage-treated foods, colonies were reisolated and tested for phage susceptibility. For this purpose, 10 Listeria colonies were randomly picked from Oxford agar plates (total of 60 clones, from trials with hot dogs, sliced turkey breast, smoked salmon, mixed seafood, cabbage, and lettuce) and streaked onto nonselective BHI plates For the phage assay, 200-μl amounts of liquid cultures of the bacterial isolates were mixed with molten soft agar and poured onto agar plates. After solidifying and drying of the agar, 10 μl of phage preparations containing 109 PFU/ml, 106 PFU/ml, and 104 PFU/ml were dropped on the plates. After incubation at 30°C for 24 h, plates were analyzed, and plaques could be counted. If no plaques occurred at all, colonies were considered to be resistant. If plaques occurred with only 1 or 2 concentrations, colonies were considered to be less sensitive and the efficiency of plating could be calculated. Colonies were considered to be fully sensitive and not resistant when there was no difference in plaque number from the number for the wild-type Listeria strain.
Statistical analysis.
Bacterial and phage counts were always determined by duplicate plating, and all experiments described here were independently performed from 2 to 5 times. Results are presented as mean values, and error bars in the figures indicate standard deviations of the means. Student's t test (unpaired, two-tailed, and heteroscedastic) was used to determine the significance of cell count differences between controls and phage-treated samples, based on an alpha-level of 5% (P = 0.05).
RESULTS
Phage A511 is effective for reducing Listeria counts.
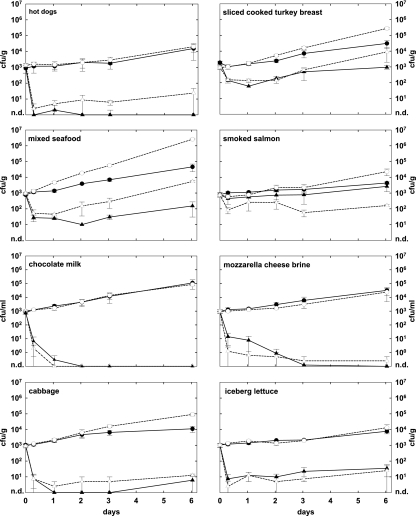
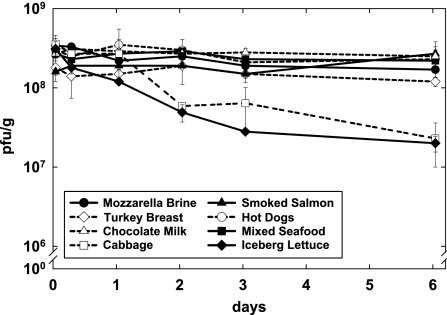
Figure 1 shows the effect of phage A511 on the growth of two different L. monocytogenes strains in eight different foods. Depending on the food substrate, Listeria counts in the nontreated controls increased by 0.7 to 3.4 log units after 6 days of incubation at 6°C. Overall, the growth rates of the two Listeria strains were similar, although Scott A grew to higher numbers in cooked turkey breast, smoked salmon, seafood, and cabbage (Fig. 1). The application of phage A511 reduced the final counts of L. monocytogenes by 0.4 to 5.0 log units (45.7 to 100%). In general, the reductions achieved by phage treatment were very similar for both Listeria strains and on most foods, except for hot dogs and smoked salmon. Here, the differences in viable counts of both strains were more than 1 log at the end of the 6-day period. On hot dogs, phage reduced the number of viable L. monocytogenes WSLC 1001 cells below the detection limit, corresponding to more than 4.2 log units (P = 0.004). With strain Scott A, this reduction was 2.9 log units (P = 0.001). On smoked salmon, the viable cells of WSLC 1001 were initially reduced by 0.8 log units (P = 0.002), but they resumed growth after 2 days. There was no significant difference after 6 days (P = 0.07). With strain Scott A, however, a reduction of 2.2 log units was observed under the same conditions (P = 0.002).
FIG. 1.
Effect of phage A511 on growth of Listeria monocytogenes strains WSLC 1001 and Scott A in eight different RTE foods. Samples were spiked with bacteria (1 × 103 CFU/g or ml), and phage A511 was applied (3 × 108 PFU/g or ml) to the test samples approximately 1 h later. Samples were stored for 6 days at 6°C and monitored for bacterial counts at time points indicated. Closed circles, WSLC 1001 controls without phage; closed triangles, WSLC 1001 with A511; open circles, Scott A controls without phage; open triangles; Scott A with phage A511; n.d., none detected.
In samples containing cabbage, iceberg lettuce, chocolate milk, and mozzarella cheese brine, phage A511 was very effective in suppressing or preventing the growth of both Listeria strains; the differences ranged from 2.3 log units (P = 0.0002) to 5.0 log units (P = 0.002) compared to the counts in the untreated controls. A more-moderate effect of phage A511 was observed on sliced cooked turkey breast and on mixed seafood, with decreases of 1.5 log units (P < 0.03) and 2.5 log units (P < 0.01), respectively.
Storage time and temperature had little effect on the efficacy of phage.
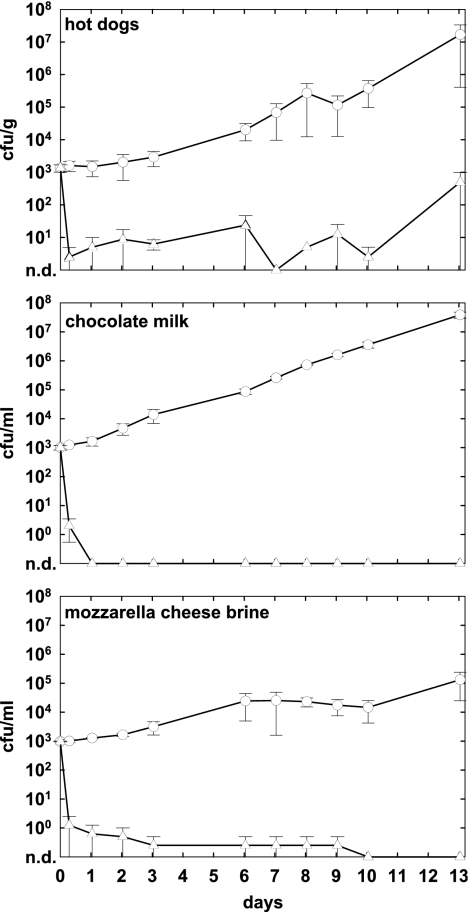
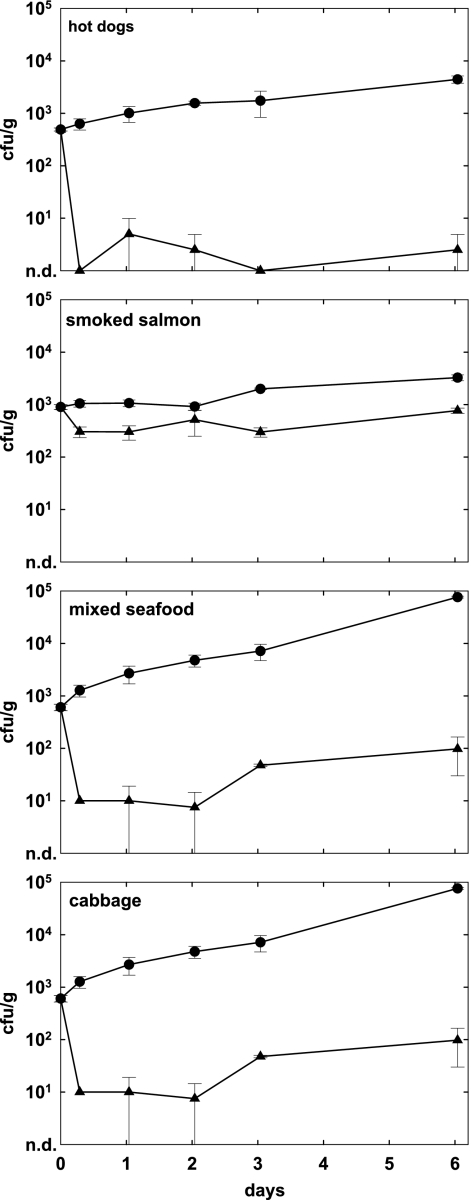
To examine the effects of phage treatment over an extended storage period reasonably applicable to fresh RTE foods, phage A511 was applied to hot dogs, chocolate milk, and mozzarella brine spiked with L. monocytogenes Scott A as described above. Foods were stored for up to 13 days at 6°C, and bacterial and phage counts were determined at regular intervals. Figure 2 shows that on hot dogs and in chocolate milk, viable counts in the controls exceeded 107 CFU/g or ml after 13 days, whereas growth was slower in mozzarella cheese brine, where cell counts reached approximately 105 CFU/ml. Phage addition to contaminated hot dogs resulted in a massive reduction of L. monocytogenes to less than 50 CFU/g. However, in one trial, a strong increase in Listeria counts was observed, which is reflected in the unusually large standard deviation of this data point. Compared to the level in the control, a reduction of 4.5 log units was achieved (P = 0.06). In chocolate milk and mozzarella cheese brine, no Listeria cells could be detected by direct plating, indicating very effective control through the phage, with 7.6-log-unit (P = 0.004) and 5.1-log (P = 0.04) differences, respectively.
FIG. 2.
Effect of phage A511 on growth of L. monocytogenes Scott A over extended storage periods. Selected foods were spiked with bacteria (1 × 103 CFU/g or ml), and A511 was applied (3 × 108 PFU/g or ml) approximately 1 h later. Samples were then stored for up to 13 days at 6°C and monitored for bacterial counts at the time points indicated. Open circles, controls without phage; open triangles, samples with A511; n.d., none detected.
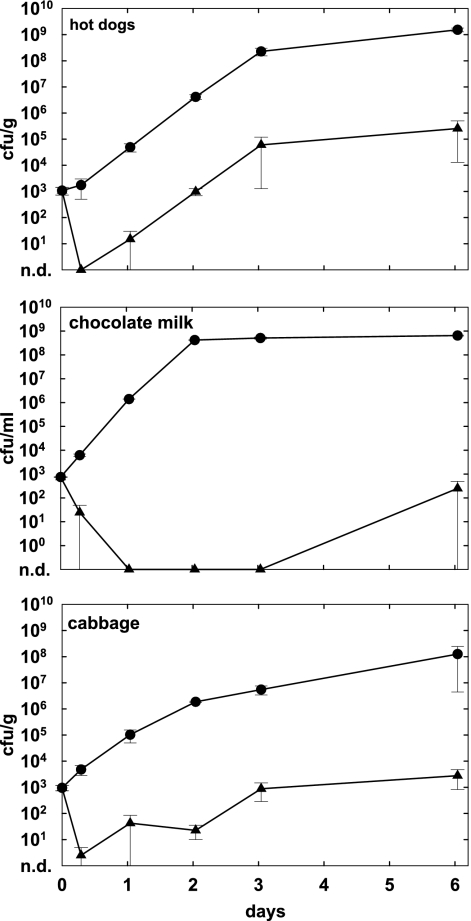
In order to examine the efficacy of phage under conditions supporting faster growth of Listeria monocytogenes, we tested phage treatment on foods stored at room temperature. Hot dogs, chocolate milk, and cabbage were contaminated with strain WSLC 1001, treated with phage A511 as described above, and subsequently stored at 20°C. Under these conditions, the generation time of the bacteria was much shorter and they grew up to 108 to 109 CFU/g or ml in the untreated controls (Fig. 3), corresponding to approximately 4 to 5 log increases over growth at 6°C. If phage was present, the differences from the controls at day 6 were similar to or higher than the results obtained at 6°C, i.e., 3.8 log units on hot dogs (P = 0.001), 4.7 log units on cabbage (P = 0.01), and 6.4 log units in chocolate milk (P < 0.0001). However, although the percent reductions were greater, the viable counts of Listeria in the phage-treated samples were also higher at 20°C.
FIG. 3.
Effect of phage A511 on growth of L. monocytogenes WSLC 1001 during storage at elevated temperature. Selected foods were spiked with bacteria (1 × 103 CFU/g or ml), and phage A511 was applied (3 × 108 PFU/g or ml) approximately 1 h later. Food samples were stored for 6 days at 20°C and monitored for bacterial counts at the time points indicated. Closed circles, controls without phage; closed triangles, samples with A511; n.d., none detected.
Effectiveness of phage is dependent on density.
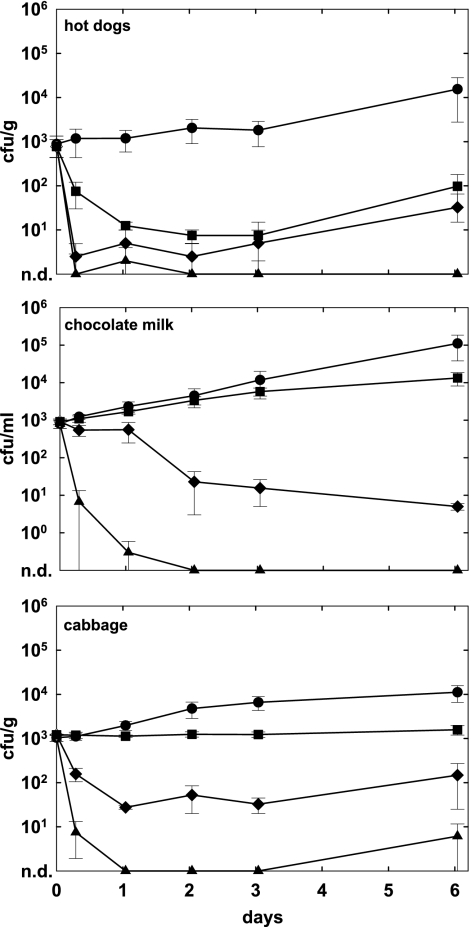
To assess the critical role of phage concentration in target cell infection and killing, we applied different doses of A511 (3 × 106 to 3 × 108 PFU/g or ml) to L. monocytogenes 1001-contaminated hot dogs, chocolate milk, and cabbage, followed by storage at 6°C for 6 days. Our results (Fig. 4) clearly demonstrate that a lower initial phage density resulted in less-significant growth suppression of L. monocytogenes. On hot dogs, the application of 3 × 108 PFU/g was sufficient to completely control outgrowth of the pathogen, whereas 3 × 107 PFU/g or 3 × 106 PFU/g reduced counts after 6 days by 2.7 and 2.2 logs, respectively (P < 0.0050). In chocolate milk, no bacteria survived when phage was used at the highest concentration. The application of lower densities (3 × 107 PFU/ml) also resulted in a massive reduction (4.4 log units; P = 0.0002), and the addition of 3 × 106 PFU/ml decreased the viable counts by 1.0 log unit (P = 0.0005). Similar results were obtained with contaminated cabbage, where 3 × 108 PFU/g also had the strongest effect (viable counts below 10 CFU/g) and lower concentrations were less effective (P < 0.0005).
FIG. 4.
Effects of different initial phage concentrations on growth inhibition of Listeria. Foods were spiked with L. monocytogenes WSLC 1001 (1 × 103 CFU/g or ml), and phage A511 was applied 1 h later at three different final concentrations (3 × 106, 3 × 107, or 3 × 108 PFU/g or ml). Samples were then stored for 6 days at 6°C and monitored for bacterial counts at the time points indicated. Closed circles, controls without phage; closed squares, 3 × 106 PFU/g phage; closed diamonds, 3 × 107 PFU/g phage; closed triangles, 3 × 108 PFU/g phage; n.d., none detected.
Phage is stable during storage.
To assess the stability and/or inactivation of phage in the food samples, we monitored infective phage (measured as PFU/g or PFU/ml) in the different foods during the course of all experiments. Figure 5 shows the phage counts for storage at 6°C over 6 days. On most foods, phage appeared very stable (maximum decrease of infectivity, 0.6 logs). In contrast, the incubation of viruses with plant products (cabbage and lettuce) resulted in a more-significant reduction of infective particles of 0.6 to 1.2 logs, up to 2.0 logs at 20°C (data not shown). However, although the phage particles were not inactivated, they apparently were immobilized relatively soon after addition to nonliquid foods and therefore became inactive due to limited diffusion (see below).
FIG. 5.
Stability of phage A511 in different RTE foods (see figure insert) during storage for 6 days at 6°C. At the time points indicated, the PFU of A511 (added at 3 × 108 PFU/g or ml) were determined directly from the food samples spiked with L. monocytogenes Scott A bacteria (1 × 103 CFU/g or ml) (see Fig. 1).
Reisolated Listeria clones remain phage sensitive.
During the course of this study, the phage sensitivity of a total of 60 Listeria isolates recovered by plating of different phage-treated foods after 6 or 13 days did not change; in other words, we could not isolate any bacteria which were insensitive to A511 in plating assays. This finding indicates that the bacteria remaining in phage-treated foods did not acquire phage resistance but rather escaped contact with phage particles during the time immediately following application, when the phage particles were able to diffuse and thus reach the target cells (see discussion below). Considering the Listeria counts monitored over the storage periods, this result also suggests that phage particles likely became immobilized in the food matrices relatively soon after addition to the foods, within 12 to 24 h.
P100 and A511 show similar efficacies.
Listeria phage P100 (10) is closely related to A511 (15, 29) and recently received GRAS status for application in all foods (5). This prompted us to evaluate and compare the effectiveness of the two phages, and we tested P100 against L. monocytogenes 1001 contamination in hot dogs, smoked salmon, seafood, and cabbage under the same experimental conditions as for A511. Overall, we found that the efficacy of P100 against Listeria (P < 0.001) (Fig. 6) was very similar to that of A511, indicating that this group of SPO1-like Listeria phages (29) is well suited for practical application in foods.
FIG. 6.
Effect of phage P100 on growth of L. monocytogenes WSLC 1001 in four different RTE foods. Samples were spiked with the bacteria (1 × 103 CFU/g or ml), and P100 was added (3 × 108 PFU/g or ml) approximately 1 h later. Samples were stored for 6 days at 6°C and monitored for bacterial counts at the time points indicated. Closed circles, controls without phage; closed triangles, samples with P100; n.d., none detected.
DISCUSSION
Our study demonstrates the usefulness of virulent bacteriophages for biocontrol of Listeria monocytogenes with a representative panel of RTE foods. The effectiveness of phages against bacterial targets can vary significantly; the difference in L. monocytogenes counts between controls and phage-treated samples ranged from less than 1 to more than 5 orders of magnitude. This indicates that successful phage infection and subsequent killing of the host cells is strongly dependent on the environmental conditions, i.e., the type of food and its specific matrix. The proportion of bacterial cells that can be infected depends on several parameters, and the two that are arguably the most important will be discussed here. First, the binding of phages to their ligands on the bacterial surfaces is influenced by intrinsic factors, such as ionic strength, pH, and substances which may interfere with this process (27). These parameters are largely defined by the food itself and may change during the production, ripening, or storage of the items. Since it is difficult (if not impossible) to predict the behavior of phages in a complex food matrix, empirical data are required.
Second, our results show that the concentration of phage at the time of application is crucial for efficacy, i.e., applying more phage generally resulted in greater inactivation. This is in accordance with the results of other studies showing that higher phage numbers yielded better results (10, 34). More specifically, our data suggest that for optimum efficacy, the phage concentration should not be less than 108 PFU/g or cm−2 at the time of application. Although this seems to be relatively high, it is both technically and economically possible. However, the application must be specifically optimized for individual food systems. The concentration of phage must be high enough to ensure the contact of the passively diffusing virus particles with their host cells, within a given time and considering spatial limitations. In liquid foods (milk and cheese brine), this does not appear to be a problem, because suspended phage particles can diffuse almost freely. The situation is different on solid foods with an even surface (hot dogs, salad leaves, etc.), where the total surface area and its ability to absorb liquid from the phage suspension are the decisive parameters. The most difficult foods to treat with phage are those with an uneven and large surface area (fish, meat, and seafood), which physically limits the distribution of phage particles in order to reach all bacterial targets. Moreover, target bacteria may be embedded within the rather complex food matrices, thereby shielding them from diffusing phage particles. Such an effect may be reflected in the results of phage treatment of precontaminated smoked salmon, seafood, and turkey meat. Considering that phage was not inactivated by these foods (Fig. 6), we conclude that limited diffusion and thus limited contact of bacteria and phage particles was responsible for the lower efficacy. This hurdle may be overcome by modifying phage application, e.g., by the use of more phage, larger liquid volumes, and/or the application of phage before bacterial contamination occurs. It should also be noted that it is necessary to use a sufficiently high phage concentration from the start, without relying on self-amplification. The burst size of A511 is approximately 40 to 50 new particles released by an infected cell (38). However, because of the generally low number of target cells present in foods, this effect cannot significantly contribute to an increase in overall phage concentration.
Testing different foods under otherwise identical conditions showed that the amount of phage required for treatment largely depends on the food matrix. Thus, protocols for the application of phage in any food production setting and environment must be individually optimized not only with respect to the phages and target organisms but also by considering specifications of the food matrix.
We found that the effect of phage was not neutralized by prolonged storage periods. A shift of the incubation temperature toward more-favorable growth conditions (20°C represents temperature abuse for most RTE foods) had little effect on the final log difference in viable counts, although the absolute Listeria CFU/g numbers were higher at the elevated temperature both in the controls and in the phage samples. This finding is in agreement with reports on other phage-host systems, where variation of storage temperature had no effect on the potential of Pseudomonas phages to extend the shelf life of raw beef (17) and the reduction of Salmonella on phage-treated honeydew melons (33).
The two strains of L. monocytogenes tested revealed no difference in their reduction by A511 treatment (P > 0.05), with the exception of smoked salmon, where strain Scott A was killed more effectively than WSLC 1001 (P < 0.05). In both sets of experiments, all other parameters were kept constant (phage concentration, food sample, storage conditions, etc.), and experiments were repeated under identical conditions. Thus, the observed differences in the fish samples appear to be strain dependent.
The efficacies of the two phages tested (A511 and P100) were also very similar. This was expected, as the two phages are both members of the Myoviridae family, share extensive nucleotide sequence homologies (10, 15, 38), and feature a broad (but still slightly different) host range within the genus Listeria (56). This particular type of SPO1-like phage (29) appears to be very suitable for the application described here.
Only limited data were available concerning the stability of phages on or in foods. Some studies report an increase of phage concentrations of 1 to 2 log units (17, 23, 40), whereas in other cases (31, 32) rapid inactivation of phages applied to fruit surfaces was reported. We found the phage particles to be quite stable in foods of animal origin. On vegetable foods (cabbage and salad), however, the concentration of infective particles decreased by approximately 1 log unit within 2 to 3 days, often accompanied by an increased Listeria cell count. Since the pH seemed not to be in a critical range, the inactivation of phage particles may be due to secondary plant compounds and substances known to inactivate viruses and bacteriophages (2, 14, 51), such as organic acids and tannins.
We did not find any bacteria isolated from phage-treated foods to be resistant against the phages used. At least under the conditions used here, insensitivity against phage A511 or P100 appears to be a rare event, most likely because of the relatively low numbers of bacterial cells encountered by the phage particles. Other researchers also failed to detect resistance against phages used to control food-borne pathogens, such as Salmonella enterica Serovar Enteritidis on fresh-cut fruit during a 7-day period (31), Listeria monocytogenes on cheese over 3 weeks (10), and Campylobacter jejuni on chicken skin after 10 days (9). However, phage-resistant Brochothrix thermosphacta emerged on pork tissue 8 days after phage treatment; 20% to 65% of the isolates revealed resistance to the phages used (24). There is no doubt that the success of using phages against bacteria will depend on the emergence or persistence of resistance against the viruses, similar to the emergence of antibiotic resistance. In order to minimize the probability that resistance will diminish the efficacy of phage treatment, several measures should be considered and adhered to: (i) the use of virulent phages with a broad host range; (ii) the application of phages with different host ranges in mixtures/cocktails, but preferably in rotating application schemes; (iii) the treatment of products immediately prior to packaging and shipment in order to prevent the reentry and establishment of a phage-resistant flora in a production environment; and (iv) strict avoidance of recycling inoculation loops (e.g., old-young smearing procedure in soft cheese production). Regarding phage resistance, it has also been shown that phage-resistant phenotypes can revert when selective forces are removed, i.e., in the absence of phage (41). However, this phenomenon is influenced by the fitness cost of phage resistance and will also be phage host dependent.
Different legal requirements and regulations exist in different countries and for different foods. With respect to Listeria in RTE foods, the United States has adopted a zero tolerance policy. In the European Union and Switzerland, up to 100 CFU/g are permitted in RTE foods which do not support growth of the pathogen to unacceptable levels until the end of the shelf life (3). Interestingly, the U.S. Food and Drug Administration has also recently published a proposal to relax the current criteria for L. monocytogenes in RTE foods not able to support growth of this pathogen, to the same level of 100 CFU/g (http://www.fda.gov/ora/compliance_ref/cpg/cpgfod/draft_cpg555-320.html).
In conclusion, we believe that the application of virulent bacteriophages for control of Listeria monocytogenes in RTE foods represents a specific, effective, and environmentally friendly path toward the production and supply of safer food. Phages may also be helpful in decontaminating food-processing equipment where L. monocytogenes may be present as a part of the individual and specific “house flora.” At this point, we are just beginning to exploit the potential of phages for combating bacterial contaminations, and the application of naturally occurring broad-host-range phages, such as A511 and P100, appears to be optimally suited for harnessing the unique properties of these natural enemies of bacteria.
Acknowledgments
We thank Steven Hagens (EBI Food Safety, Wageningen, The Netherlands) for providing P100 bacteriophage and for helpful discussions and Martina Haug for technical assistance.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Abuladze, T., M. Li, M. Y. Menetrez, T. Dean, A. Senecal, and A. Sulakvelidze. 2008. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 74:6230-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad, A., S. F. Asad, S. Singh, and S. M. Hadi. 2000. DNA breakage by resveratrol and Cu(II): reaction mechanism and bacteriophage inactivation. Cancer Lett. 154:29-37. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2005. Commission regulation (EC) no. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs, p.L338/1-L338/26, vol. 2073/2005. European Commission, Brussels, Belgium.
- 4.Anonymous. Datenquelle “SurvStat@RKI.” Robert Koch Institut. http://www3.rki.de/SurvStat/QueryForm.aspx. Accessed 24 November 2008.
- 5.Anonymous. 22 June 2007. GRAS notice no. GRN 000218. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration. http://www.cfsan.fda.gov/∼rdb/opa-g218.html.
- 6.Anonymous. Meldesystem Infektionskrankheiten, Listeria. Bundesamt für Gesundheit. www.bag.admin.ch/infreporting/tab/tc13.htm. Accessed 24 November 2008.
- 7.Anonymous. 1997. EN ISO 11290: microbiology of food and animal feeding stuffs. Horizontal method for the detection and enumeration of Listeria monocytogenes. Part 1:1997, detection method. International Organization for Standardization, Geneva, Switzerland.
- 8.Anonymous. 1995. IDF standard 143A:1995, milk and milk products. Detection of Listeria monocytogenes. International Dairy Federation, Brussels, Belgium.
- 9.Atterbury, R. J., P. L. Connerton, C. E. Dodd, C. E. Rees, and I. F. Connerton. 2003. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6302-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlton, R. M., W. H. Noordman, B. Biswas, E. D. de Meester, and M. J. Loessner. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43:301-312. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2006. Notifiable diseases/deaths in selected cities. Weekly information. MMWR Morb. Mortal. Wkly. Rep. 55:1157. [Google Scholar]
- 12.Chibani-Chennoufi, S., A. Bruttin, M. L. Dillmann, and H. Brüssow. 2004. Phage-host interaction: an ecological perspective. J. Bacteriol. 186:3677-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton, C. B., C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor, and P. M. Griffin. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 336:100-105. [DOI] [PubMed] [Google Scholar]
- 14.de Siqueira, R. S., C. E. Dodd, and C. E. Rees. 2006. Evaluation of the natural virucidal activity of teas for use in the phage amplification assay. Int. J. Food Microbiol. 111:259-262. [DOI] [PubMed] [Google Scholar]
- 15.Dorscht, J. 2007. Comparative genomics of Listeria bacteriophages. Dissertation. Technische Universität München, Weihenstephan-Freising, Germany.
- 16.Dykes, G. A., and S. M. Moorhead. 2002. Combined antimicrobial effect of nisin and a listeriophage against Listeria monocytogenes in broth but not in buffer or on raw beef. Int. J. Food Microbiol. 73:71-81. [DOI] [PubMed] [Google Scholar]
- 17.Ellis, D. E., P. A. Whitman, and R. T. Marshall. 1973. Effects of homologous bacteriophage on growth of Pseudomonas fragi WY in milk. Appl. Microbiol. 25:24-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming, D. W., S. L. Cochi, K. L. MacDonald, J. Brondum, P. S. Hayes, B. D. Plikaytis, M. B. Holmes, A. Audurier, C. V. Broome, and A. L. Reingold. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312:404-407. [DOI] [PubMed] [Google Scholar]
- 19.Goode, D., V. M. Allen, and P. A. Barrow. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69:5032-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb, S. L., E. C. Newbern, P. M. Griffin, L. M. Graves, R. M. Hoekstra, N. L. Baker, S. B. Hunter, K. G. Holt, F. Ramsey, M. Head, P. Levine, G. Johnson, D. Schoonmaker-Bopp, V. Reddy, L. Kornstein, M. Gerwel, J. Nsubuga, L. Edwards, S. Stonecipher, S. Hurd, D. Austin, M. A. Jefferson, S. D. Young, K. Hise, E. D. Chernak, and J. Sobel. 2006. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin. Infect. Dis. 42:29-36. [DOI] [PubMed] [Google Scholar]
- 21.Graves, L. M., S. B. Hunter, A. R. Ong, D. Schoonmaker-Bopp, K. Hise, L. Kornstein, W. E. DeWitt, P. S. Hayes, E. Dunne, P. Mead, and B. Swaminathan. 2005. Microbiological aspects of the investigation that traced the 1998 outbreak of listeriosis in the United States to contaminated hot dogs and establishment of molecular subtyping-based surveillance for Listeria monocytogenes in the PulseNet network. J. Clin. Microbiol. 43:2350-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greer, G. G. 2005. Bacteriophage control of foodborne bacteria. J. Food Prot. 68:1102-1111. [DOI] [PubMed] [Google Scholar]
- 23.Greer, G. G. 1986. Homologous bacteriophage control of Pseudomonas growth and beef spoilage. J. Food Prot. 49:104-109. [DOI] [PubMed] [Google Scholar]
- 24.Greer, G. G., and B. D. Dilts. 2002. Control of Brochothrix thermosphacta spoilage of pork adipose tissue using bacteriophages. J. Food Prot. 65:861-863. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson, D. A. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312-323. [DOI] [PubMed] [Google Scholar]
- 26.Hudson, J. A., C. Billington, G. Carey-Smith, and G. Greening. 2005. Bacteriophages as biocontrol agents in food. J. Food Prot. 68:426-437. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy, J. E., and G. Bitton. 1987. Bacteriophages in foods, p. 289-316. In S. M. Goyal, C. P. Gerba, and G. Bitton (ed.), Phage ecology. John Wiley and Sons, New York, NY.
- 28.Kim, K. P., J. Klumpp, and M. J. Loessner. 2007. Enterobacter sakazakii bacteriophages can prevent bacterial growth in reconstituted infant formula. Int. J. Food Microbiol. 115:195-203. [DOI] [PubMed] [Google Scholar]
- 29.Klumpp, J., J. Dorscht, R. Lurz, R. Bielmann, M. Wieland, M. Zimmer, R. Calendar, and M. J. Loessner. 2008. The terminally redundant, nonpermuted genome of Listeria bacteriophage A511: a model for the SPO1-like myoviruses of gram-positive bacteria. J. Bacteriol. 190:5753-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer, P., M. Y. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leverentz, B., W. S. Conway, Z. Alavidze, W. J. Janisiewicz, Y. Fuchs, M. J. Camp, E. Chighladze, and A. Sulakvelidze. 2001. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J. Food Prot. 64:1116-1121. [DOI] [PubMed] [Google Scholar]
- 32.Leverentz, B., W. S. Conway, M. J. Camp, W. J. Janisiewicz, A. Abuladze, M. Yang, R. Saftner, and A. Sulakvelidze. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leverentz, B., W. S. Conway, W. Janisiewicz, M. Abadias, C. P. Kurtzman, and M. J. Camp. 2006. Biocontrol of the food-borne pathogens Listeria monocytogenes and Salmonella enterica serovar Poona on fresh-cut apples with naturally occurring bacterial and yeast antagonists. Appl. Environ. Microbiol. 72:1135-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leverentz, B., W. S. Conway, W. Janisiewicz, and M. J. Camp. 2004. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67:1682-1686. [DOI] [PubMed] [Google Scholar]
- 35.Loc Carrillo, C., R. J. Atterbury, A. El-Shibiny, P. L. Connerton, E. Dillon, A. Scott, and I. F. Connerton. 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71:6554-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loessner, M. J., and M. Busse. 1990. Bacteriophage typing of Listeria species. Appl. Environ. Microbiol. 56:1912-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loessner, M. J., M. Rudolf, and S. Scherer. 1997. Evaluation of luciferase reporter bacteriophage A511::luxAB for detection of Listeria monocytogenes in contaminated foods. Appl. Environ. Microbiol. 63:2961-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loessner, M. J., and S. Scherer. 1995. Organization and transcriptional analysis of the Listeria phage A511 late gene region comprising the major capsid and tail sheath protein genes cps and tsh. J. Bacteriol. 177:6601-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modi, R., Y. Hivri, A. Hill, and M. W. Griffiths. 2001. Effect of phage on survival of Salmonella enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. J. Food Prot. 64:927-933. [DOI] [PubMed] [Google Scholar]
- 41.O'Flynn, G., R. P. Ross, G. F. Fitzgerald, and A. Coffey. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70:3417-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen, S. J., M. Patrick, S. B. Hunter, V. Reddy, L. Kornstein, W. R. MacKenzie, K. Lane, S. Bidol, G. A. Stoltman, D. M. Frye, I. Lee, S. Hurd, T. F. Jones, T. N. LaPorte, W. Dewitt, L. Graves, M. Wiedmann, D. J. Schoonmaker-Bopp, A. J. Huang, C. Vincent, A. Bugenhagen, J. Corby, E. R. Carloni, M. E. Holcomb, R. F. Woron, S. M. Zansky, G. Dowdle, F. Smith, S. Ahrabi-Fard, A. R. Ong, N. Tucker, N. A. Hynes, and P. Mead. 2005. Multistate outbreak of Listeria monocytogenes infection linked to delicatessen turkey meat. Clin. Infect. Dis. 40:962-967. [DOI] [PubMed] [Google Scholar]
- 43.Pao, S., S. P. Randolph, E. W. Westbrook, and H. Shen. 2004. Use of bacteriophages to control Salmonella in experimentally contaminated sprout seeds. J. Food Sci. 69:127-130. [Google Scholar]
- 44.Rees, C. E., and C. E. Dodd. 2006. Phage for rapid detection and control of bacterial pathogens in food. Adv. Appl. Microbiol. 59:159-186. [DOI] [PubMed] [Google Scholar]
- 45.Ryser, E. T. 1999. Foodborne listeriosis, p. 299-358. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis and food safety, 2nd ed. Marcell Dekker, Inc., New York, NY.
- 46.Schlech, W. F. 2000. Foodborne listeriosis. Clin. Infect. Dis. 31:770-775. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz, B., C. A. Ciesielski, C. V. Broome, S. Gaventa, G. R. Brown, B. G. Gellin, A. W. Hightower, and L. Mascola. 1988. Association of sporadic listeriosis with consumption of uncooked hot dogs and undercooked chicken. Lancet ii:779-782. [DOI] [PubMed] [Google Scholar]
- 48.Seeliger, H. P. R., and D. Jones. 1986. Listeria, p. 1235-1245. Bergey's manual of systematic bacteriology, vol. 1. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 49.Siegman-Igra, Y., R. Levin, M. Weinberger, Y. Golan, D. Schwartz, Z. Samra, H. Konigsberger, A. Yinnon, G. Rahav, N. Keller, N. Bisharat, J. Karpuch, R. Finkelstein, M. Alkan, Z. Landau, J. Novikov, D. Hassin, C. Rudnicki, R. Kitzes, S. Ovadia, Z. Shimoni, R. Lang, and T. Shohat. 2002. Listeria monocytogenes infection in Israel and review of cases worldwide. Emerg. Infect. Dis. 8:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slutzker, L., and A. Schuchat. 1999. Listeriosis in humans, p. 75-95. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis and food safety, vol. 2. Marcell Dekker, Inc., New York, NY. [Google Scholar]
- 51.Stewart, G. S., S. A. Jassim, S. P. Denyer, P. Newby, K. Linley, and V. K. Dhir. 1998. The specific and sensitive detection of bacterial pathogens within 4 h using bacteriophage amplification. J. Appl. Microbiol. 84:777-783. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Boland, J. A., M. Kuhn, P. Bercher, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whichard, J. M., N. Sriranganathan, and F. W. Pierson. 2003. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J. Food Prot. 66:220-225. [DOI] [PubMed] [Google Scholar]
- 54.Whitman, P. A., and R. T. Marshall. 1971. Characterization of two psychrophilic Pseudomonas bacteriophages isolated from ground beef. Appl. Microbiol. 22:463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitman, P. A., and R. T. Marshall. 1971. Isolation of psychrophilic bacteriophage-host systems from refrigerated food products. Appl. Microbiol. 22:220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zink, R., and M. J. Loessner. 1992. Classification of virulent and temperate bacteriophages of Listeria spp. on the basis of morphology and protein analysis. Appl. Environ. Microbiol. 58:296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]