Abstract
A freshly isolated oral strain, Lactobacillus casei RB1014, was grown in continuous culture to compare the effects of pH and fluoride on growth and metabolism. The cells were grown at pH 7.0 to 3.2 in the absence of fluoride and from pH 7.0 to 5.4 with 20 mM NaF. Cell numbers varied from 3 X 10(9) to 30 X 10(9)/ml on blood agar during alterations in the growth pH from 7.0 to 4.27. Only when the culture was stressed by lowering the pH to 3.2 were cell numbers drastically reduced. Cells growing at pH 7.0 without fluoride were unable to grow when plated on fluoride agar (10.5 mM) at pH 5.5; however, when the growth pH was allowed to decrease to 4.94, cells grew on the fluoride plates in numbers equal to those growing on blood agar. This fluoride tolerance trait appeared rapidly once pH control was removed and was lost when the culture was returned to pH 7.0. The addition of 20 mM NaF to the culture medium did not adversely affect growth, provided that the pH was maintained at 6.0 or above; cells tolerant to 10.5 and 16 mM NaF appeared on pH 5.5 plates during this phase. In cells removed from the chemostat throughout the experiment and incubated at the pH of growth in a pH stat, glycolytic activity was optimum at pH 5.5 in the absence of NaF. Fluoride stimulated glycolytic activity by cells incubated at pH 7.0 and by cells growing with 20 mM NaF, provided that the pH of growth remained at or above 6.0. A more detailed examination of the adaptation to fluoride tolerance during shifts to acidic pH values revealed that cells capable of growth on acidic fluoride agar plates appeared within 2 h of the start of the fall in pH of the chemostat culture. Estimation of the intracellular pH during the period of the initial pH fall revealed that the intracellular pH was identical to the extracellular pH (i.e., no pH gradient [delta pH]), indicating that fluoride would not be transported into the cells to inhibit metabolism. However, once the pH of the medium was stabilized, delta pHs were generated, with the delta pH increasing as the pH declined. The inhibition of glycolysis by fluoride increased in proportion to the delta pH. Cells grown at pH 5.5 generated larger delta pHs than did cells grown at pH 7.0, although the values were normally small (approximately 0.9 U). The data suggest that the inherent fluoride tolerance of L. casei RB1014 was associated with relatively small delta pHs.
Full text
PDF

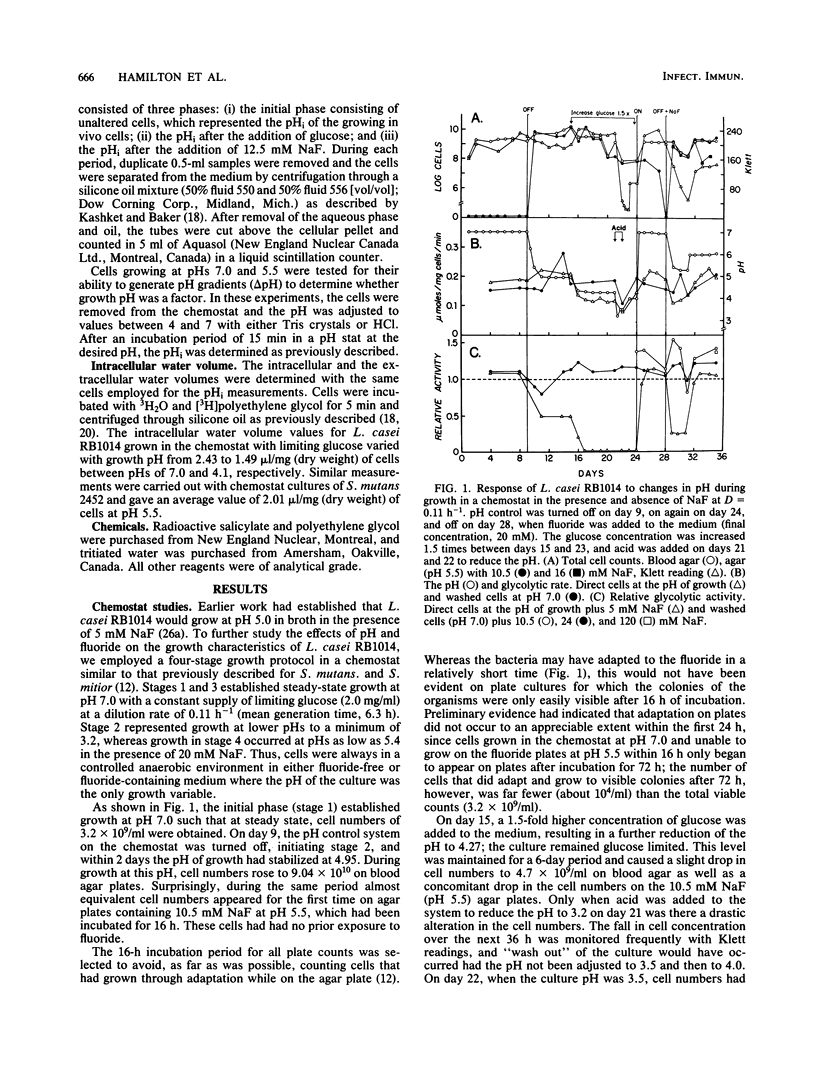

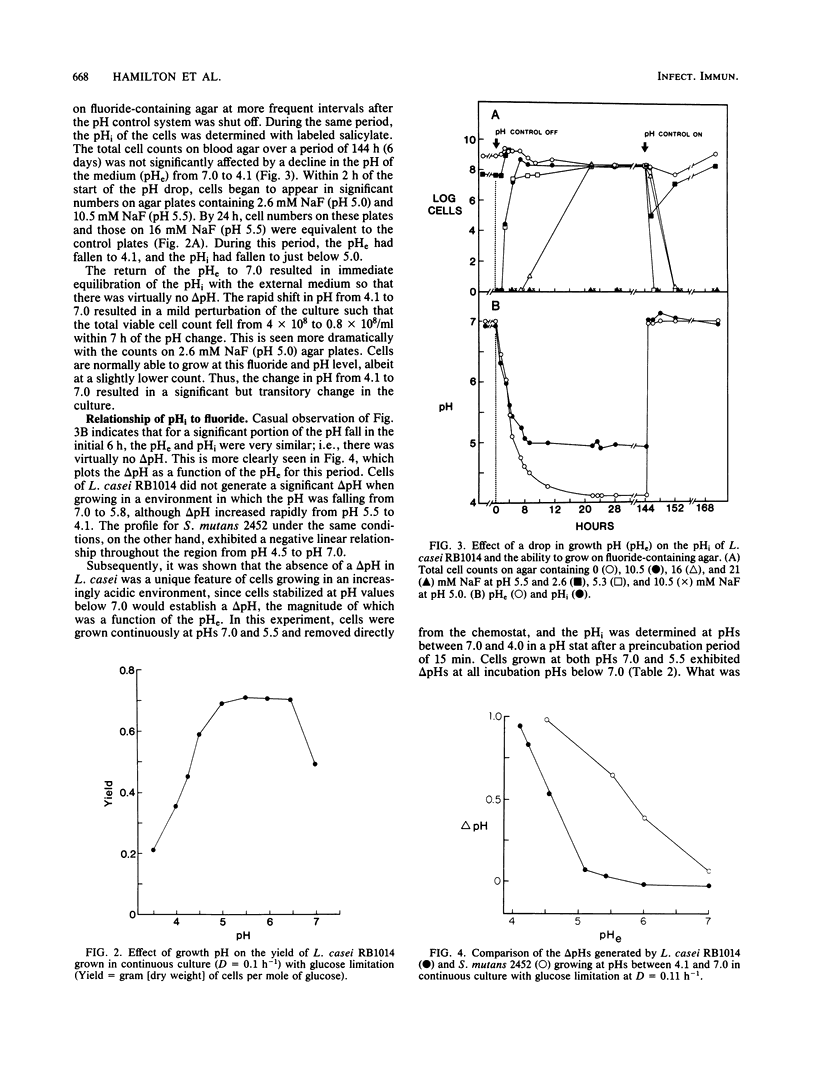


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. H., Odlum O., Nolette N., Hamilton I. R. Microbial populations growing in the presence of fluoride at low pH isolated from dental plaque of children living in an area with fluoridated water. Infect Immun. 1982 Apr;36(1):247–254. doi: 10.1128/iai.36.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRKSEN T. R., LITTLE M. F., BIBBY B. G. The pH of carious cavities-II. The pH at different depths in isolated cavities. Arch Oral Biol. 1963 Mar-Apr;8:91–97. doi: 10.1016/0003-9969(63)90046-3. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Bacteriological studies on deep areas of carious dentine. Odontol Revy Suppl. 1974;32:1–143. [PubMed] [Google Scholar]
- Eisenberg A. D., Marquis R. E. Enhanced transmembrane proton conductance in Streptococcus mutans GS-5 due to ionophores and fluoride. Antimicrob Agents Chemother. 1981 May;19(5):807–812. doi: 10.1128/aac.19.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Bowden G. H. Response of freshly isolated strains of Streptococcus mutans and Streptococcus mitior to change in pH in the presence and absence of fluoride during growth in continuous culture. Infect Immun. 1982 Apr;36(1):255–262. doi: 10.1128/iai.36.1.255-262.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huis in 't Veld J. H., van Palenstein Helderman W. H., Dirks O. B. Streptococcus mutans and dental caries in humans: a bacteriological and immunological study. Antonie Van Leeuwenhoek. 1979;45(1):25–33. doi: 10.1007/BF00400775. [DOI] [PubMed] [Google Scholar]
- Huxley H. G. The cariogenicity of dietary sucrose at variaous levels in two strains of rat under unrestricted and controlled-frequency feeding conditions. Caries Res. 1977;11(4):237–242. doi: 10.1159/000260274. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Jenkins G. N., Edgar W. M. Distribution and forms of F in saliva and plaque. Caries Res. 1977;11 (Suppl 1):226–242. doi: 10.1159/000260302. [DOI] [PubMed] [Google Scholar]
- KINGSLEY G. R., GETCHELL G. Direct ultramicro glucose oxidase method for determination of glucose in biologic fluids. Clin Chem. 1960 Oct;6:466–475. [PubMed] [Google Scholar]
- Kashket E. R., Barker S. L. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J Bacteriol. 1977 Jun;130(3):1017–1023. doi: 10.1128/jb.130.3.1017-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket S., Rodriguez V. M. Fluoride accumulation by a strain of human oral Streptococcus sanguis. Arch Oral Biol. 1976;21(8):459–464. doi: 10.1016/0003-9969(76)90103-5. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Hamilton I. R. Comparison of polyvinyl chloride membrane electrodes sensitive to alkylphosphonium ions for the determination of the electrical difference (delta psi) of Streptococcus mutans and Lactobacillus casei. Anal Biochem. 1984 May 15;139(1):228–236. doi: 10.1016/0003-2697(84)90410-x. [DOI] [PubMed] [Google Scholar]
- Kozousková J., Kubániová Z., Nováková M. Príspevek k vlivu fluóru v lokální aplikaci na nekteré skupiny ústních mikroorganismů. Cesk Stomatol. 1966 Sep;66(5):326–331. [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. The predominant cultivable flora of carious plaque and carious dentine. Caries Res. 1973;7(3):201–216. doi: 10.1159/000259844. [DOI] [PubMed] [Google Scholar]
- Maltz M., Emilson C. G. Susceptibility of oral bacteria to various fluoride salts. J Dent Res. 1982 Jun;61(6):786–790. doi: 10.1177/00220345820610062701. [DOI] [PubMed] [Google Scholar]
- Mikkelsen L., Jensen S. B., Jakobsen J. Microbial studies on plaque from carious and caries-free proximal tooth surfaces in a population with high caries experience. Caries Res. 1981;15(5):428–435. doi: 10.1159/000260548. [DOI] [PubMed] [Google Scholar]
- Newbrun E. Achievements of the seventies: community and school fluoridation. J Public Health Dent. 1980 Summer;40(3):234–247. doi: 10.1111/j.1752-7325.1980.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Reussner G. H., Galimidi A., Coccodrilli G., Jr Effects of calcium on smooth surface carious lesions in rats. J Dent Res. 1977 Jan;56(1):90–90. doi: 10.1177/00220345770560011801. [DOI] [PubMed] [Google Scholar]
- STRALFORS A. Investigations into the bacterial chemistry of dental plaques. Odontol Tidskr. 1950;58(3-4):152–341. [PubMed] [Google Scholar]
- Weatherell J. A., Deutsch D., Robinson C., Hallsworth A. S. Assimilation of fluoride by enamel throughout the life of the tooth. Caries Res. 1977;11 (Suppl 1):85–115. doi: 10.1159/000260297. [DOI] [PubMed] [Google Scholar]
- Whitford G. M., Schuster G. S., Pashley D. H., Venkateswarlu P. Fluoride uptake by Streptococcus mutans 6715. Infect Immun. 1977 Dec;18(3):680–687. doi: 10.1128/iai.18.3.680-687.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


