Abstract
Bacillus anthracis is surrounded by a capsular polypeptide composed of poly-γ-d-glutamic acid (PGA). This antiphagocytic capsule is an essential virulence factor and is shed into body fluids during a murine model of pulmonary anthrax. Our previous studies of a murine model for antigen clearance showed that purified PGA accumulates in the liver and spleen, most notably in splenic macrophages and the Kupffer cells and sinusoidal endothelial cells of the liver. Although the tissue and cellular depots have been identified, there is little known about the uptake and intracellular fate of PGA. As a consequence, we examined the cellular uptake and organelle localization of PGA in the murine macrophage-like cell line J774.2. We found that PGA binds to and is internalized by J774.2 cells and accumulates in CD71 transferrin receptor-positive endosomes. The receptor-mediated endocytosis inhibitors amantadine and phenylarsine oxide inhibited the binding and uptake of PGA in these cells. Cytochalasin D and vinblastine, actin and microtubule inhibitors, respectively, failed to completely inhibit binding and uptake. Finally, we found that PGA is degraded in J774.2 cells starting 4 h after uptake, with continued degradation occurring for at least 24 h. This degradation of PGA may explain the rapid clearance of PGA that is observed in vivo compared to the slow clearance noted with capsular polysaccharides.
Bacillus anthracis, the causative agent of anthrax, is surrounded by an antiphagocytic capsule that is an essential virulence factor (7, 13, 32). The capsule is unusual because it is composed of poly-γ-d-glutamic acid (PGA) (12); encapsulated bacteria are typically surrounded by a polysaccharide capsule. PGA is shed into body fluids in high concentrations during a murine model of pulmonary anthrax (15). However, our previous studies also found that purified PGA is rapidly cleared from the blood (24 h) in mice (28). This rapid clearance contrasts with the much slower in vivo clearance of capsular polysaccharides which remain in the blood for several days (11, 28). PGA is also rapidly cleared from tissues, with the complete clearance of measurable antigen after 21 days, and excreted into the urine as fragments of heterogeneous size (28). In contrast, Kaplan et al. found that pneumococcal polysaccharide remains in murine tissues up to 75 days (14).
Previous studies of a murine model of antigen clearance showed that purified PGA accumulates in the liver, specifically in the Kupffer cells and the sinusoidal endothelial cells (28), with smaller amounts of PGA in the splenic macrophages. However, the intracellular location and kinetics for the uptake of PGA by host cells are not known. Macrophages ingest particles and macromolecules via several different pathways, including phagocytosis, pinocytosis, and receptor-mediated endocytosis (5). Although each of these pathways may be unique to the object being endocytosed, once inside, the general trafficking pathways are similar. Typically, molecules are endocytosed and taken from early endosomes to late endosomes and onto the lysosome for degradation (22). Although most molecules follow this pathway, some proteins, including transferrin, are recycled back to the plasma membrane via the recycling endosomal pathway (1). In addition, some proteins, such as cholera toxin, undergo retrograde transport from the early endosomes, back to the trans-Golgi network (25, 26).
In an attempt to better understand the intracellular trafficking of PGA, we examined the kinetics for uptake and the intracellular location of PGA in the macrophage-like cell line J774.2. In addition, microtubule, actin, and receptor-mediated endocytosis inhibitors were used to examine the potential mechanisms for PGA binding and uptake. Glucuronoxylomannan (GXM), the capsular polysaccharide from Cryptococcus neoformans, was used as a model for comparison of the uptake of polypeptide versus polysaccharide capsular antigens. Our results show that PGA is taken up and trafficked through the recycling endosomes; such transport can be blocked by inhibitors of receptor-mediated endocytosis.
MATERIALS AND METHODS
Cell lines and reagents.
The murine macrophage-like cell line J774.2 (Sigma, St. Louis, MO) was used for all studies. Cells were maintained in either Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and 100 μg/ml of kanamycin (Mediatech, Herndon, VA) or RPMI with 15% fetal bovine serum in T-75 culture flasks at 37°C with 5% CO2. The cells were used between 10 and 25 passages. PGA from B. anthracis Pasteur was isolated as previously described (28). Anti-PGA monoclonal antibodies (MAbs) were generated as previously described (15). GXM, the major capsular polysaccharide of C. neoformans, was isolated as previously described (3). MAb 3C2 was used for the staining of GXM. MAbs were fluorescently labeled with Alexa Fluor 488 or 555 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. MAbs were also labeled with horseradish peroxidase according to the manufacturer's instructions using the EZ-Link Plus activated peroxidase kit (Pierce, Rockford, IL).
Kinetics for PGA internalization.
J774.2 cells were seeded at 2.0 × 105 cells/well on 8-well chamber slides (Nalge; Nunc, Naperville, IL) and grown overnight at 37°C with 5% CO2. The cells were washed once with phosphate-buffered saline (PBS) to remove nonadherent cells and incubated with PGA (100 μg/300 μl) for 15 min at room temperature to allow for initial binding. The cells were washed three times with PBS to remove the unbound PGA and were further incubated from 15 min to 24 h at 37°C to allow for the internalization of bound PGA. The cells were then fixed with 2% paraformaldehyde in PBS for 10 min on ice followed by three washes with PBS. To examine the time course for internalization, fixed cells were blocked with 1% bovine serum albumin (BSA) in PBS for 30 min, and the exterior PGA was stained by incubation for 30 min with Alexa Fluor 555 MAb F24F2 (5 μg/ml 1% BSA in PBS [blocking buffer]). The cells were then permeabilized for 30 min at room temperature with 0.1% saponin, washed, and incubated for 30 min with blocking buffer. The interior PGA was then stained by incubation for 30 min with Alexa Fluor 488-labeled MAb F26G3 (5 μg/ml blocking buffer). The cells were washed three times with PBS, coverslipped using Aquamount, and dried overnight. Images were obtained with a Nikon Eclipse E800 microscope fitted with a Nikon C1 confocal system. The images were cropped using Simple PCI 5.1 (Compix, Inc., Sewickley, PA).
Organelle localization.
The cells were seeded on eight-well chamber slides as described above, washed, and incubated with PGA for the times indicated in Fig. 2. For the identification of the Golgi apparatus, the cells were incubated for 30 min at 37°C with Alexa Fluor 555-labeled cholera toxin subunit B (5 μg/ml culture medium) (Invitrogen, Carlsbad, CA) to allow for trafficking to the organelle. The cells were fixed with 2% paraformaldehyde in PBS for 10 min on ice followed by washing with PBS. The cells were then permeabilized and blocked as described above. For all organelle localization studies, exterior-bound PGA was blocked by incubating the cells for 30 min with unlabeled MAb F24F2 (5 μg/ml) prior to permeabilization. The lysosome was labeled by incubation for 30 min with LAMP-1 antibody (5 μg/ml) (Abcam, Cambridge, MA) followed by Alexa Fluor 555-labeled goat anti-rat immunoglobulin G (IgG) (1:400) (Invitrogen, Carlsbad, CA). The recycling and early endosomes were labeled with anti-CD71 (1:800) (Santa Cruz Biotechnology, Santa Cruz, CA) followed by Alexa Fluor 488 or 555-labeled goat anti-rat IgG (1:400).
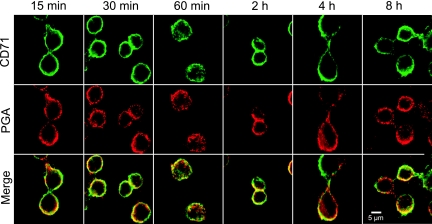
FIG. 2.
Organelle localization of PGA in J774.2 cells. Cells were incubated with PGA for the indicated time points. Exterior bound PGA was blocked using unlabeled anti-PGA MAb. Cells were permeabilized and labeled for CD71-positive endosomes (green) and PGA (red). CD71 was labeled with rat anti-CD71 followed by Alexa Fluor 488-labeled goat anti-rat IgG. PGA was stained with Alexa Fluor 555-labeled MAb F24F2. The bottom row shows the colocalization of PGA to CD71-positive endosomes (yellow). The results are representative of five experiments with similar results.
Treatment with inhibitors.
Amantadine, phenylarsine oxide, cytochalasin D, and vinblastine were purchased from Sigma (St. Louis, MO). Amantadine and vinblastine were dissolved in sterile water; phenylarsine oxide and cytochalasin D were dissolved in dimethyl sulfoxide. The working concentrations for each inhibitor were as follows: amantadine, 0.1, 0.5, and 1 mg/ml; phenylarsine oxide, 0.1 and 0.5 μg/ml; cytochalasin D, 0.1, 0.5, and 5 μM; and vinblastine, 1, 5, and 50 μM. All inhibitors were diluted in warm Dulbecco's modified Eagle's medium, and the cells were pretreated with inhibitors for 30 min at 37°C. After the pretreatment, PGA (100 μg/300 μl), GXM (50 μg/ml), or Alexa Fluor 555-labeled transferrin (5 μl/300 μl medium) was added, and the cells were incubated with PGA or transferrin for 1 h or GXM for 2 h at 37°C. The cells were fixed with 2% paraformaldehyde for 10 min at 4°C, washed, and blocked with 1% BSA in PBS. The cells were then labeled as described above with Alexa Fluor 555-labeled MAb F24F2 (exterior PGA) or Alexa Fluor 555-labeled MAb 3C2 (5 μg/ml) (exterior GXM) and Alexa Fluor 488-labeled MAb F26G3 (interior PGA) or Alexa Fluor 488-labeled MAb 3C2 (5 μg/ml) (interior GXM). Transferrin alone (5 μl/300 μl medium), GXM alone (50 μg/ml), or PGA alone (100 μg/300 μl) was used as a control in all experiments. The cells were examined for viability over a range of inhibitor concentrations using trypan blue; the cells were found to be more than 80% viable at all working concentrations of inhibitors.
Molecular sieve chromatography.
A Superdex 200 molecular sieve (GE Healthcare, Piscataway, NJ) with a running buffer of PBS-Tween 20 (0.05%) was used to compare the molecular sizes of native PGA and PGA from macrophage cell lysates. J774.2 cells (2.0 × 105) were incubated with PGA (100 μg/300 μl) for 30 min, 1 h, 2 h, 4 h, 8 h, and 24 h. The cells were then lysed using 1% Tween 20 in sterile water and cleared by centrifugation for 10 min at 5,000 rpm. The concentrations of PGA in the supernatant fluids from the cell lysates were determined by antigen capture enzyme-linked immunosorbent assay (ELISA) in comparison to a standard of purified, native PGA. The column was loaded with 50 ng of PGA diluted in 500 μl of running buffer. The relative amount of PGA in the fractions was determined by ELISA, with the results reported as the optical density at 450 nm (OD450) for each fraction.
RESULTS
Internalization of PGA by J774.2 cells.
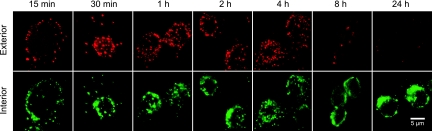
Previous studies in our laboratory found that intravenously injected PGA is taken up by macrophages in the liver and spleen. To assess PGA-macrophage interactions further, we examined the kinetics for the uptake of PGA by J774.2 cells, a murine macrophage-like cell line. Exterior and interior PGA was identified over the indicated time course by the use of fluorescently labeled MAbs to PGA (Fig. 1). Although a portion of the PGA is internalized at all time points shown, a detectable amount of PGA remained bound to the exterior of the cell as far out as 8 h; however, PGA was completely internalized after 24 h (Fig. 1).
FIG. 1.
Kinetics of the uptake of PGA by J774.2 cells. J774.2 cells were incubated for 15 min with PGA (100 μg/300 μl), washed, and further incubated for the indicated times. Exterior PGA was labeled with Alexa 555-labeled F24F2 (red), and interior PGA was labeled with Alexa 488-labeled F26G3 (green). The results are representative of five experiments with similar results.
Organelle localization.
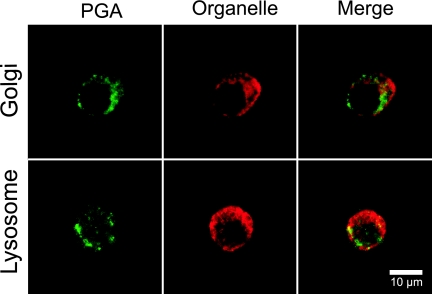
Once PGA internalization was verified, we examined PGA distribution to organelles as a function of time. PGA localized to CD71 transferrin receptor-positive recycling endosomes at all time points tested (Fig. 2). PGA also localized to early endosomal antigen 1 (EEA-1)-positive endosomes (data not shown). While a majority of the PGA localized to the recycling endosomes, a small portion also localized to the lysosome at incubation times that ranged from 30 min to 8 h (Fig. 3 and data not shown). Figure 3 shows a representative time point of 1 h of incubation with PGA. PGA did not localize to the Golgi apparatus (Fig. 3).
FIG. 3.
Lysosome and Golgi apparatus localization in J774.2 cells. Cells were incubated for 1 h with PGA. PGA bound to the cell exterior was blocked by incubation with unlabeled anti-PGA MAb. The Golgi apparatus was localized by coincubation with Alexa Fluor 555-labeled cholera toxin subunit B (top panel, red). The cells were fixed and permeabilized, and the lysosomes were stained with rat LAMP-1 and Alexa Fluor 555-labeled goat anti-rat IgG (bottom panel, red). PGA in the cell interior was stained with Alexa Fluor 488-labeled F26G3 (green). The results are representative of three experiments with similar results.
Inhibition studies.
We used several inhibitors to examine how PGA is taken up by J774.2 cells. Amantadine and phenylarsine are inhibitors of receptor-mediated endocytosis (9, 18). Both amantadine and phenylarsine oxide inhibited PGA binding and uptake at 0.5 mg/ml and 0.5 μg/ml, respectively (Fig. 4). As a positive control, amantadine (1 mg/ml) and phenylarsine oxide (0.5 μg/ml) blocked the uptake of transferrin, an iron transporter found in recycling endosomes (Fig. 4). The actin inhibitor cytochalasin D produced considerable but incomplete inhibition of PGA binding and internalization at 0.5 μM (Fig. 5). Vinblastine, an inhibitor of microtubule assembly, failed to inhibit PGA binding and internalization at 1, 5, and 50 μM concentrations (Fig. 5 and data not shown). In contrast, the binding of the capsular polysaccharide GXM was inhibited by both vinblastine and cytochalasin D (Fig. 5), a result that is in agreement with previous studies reported by Chang et al. (2). Transferrin uptake was not blocked by either vinblastine or cytochalasin D (data not shown).
FIG. 4.
Effects of inhibitors of receptor-mediated endocytosis on PGA binding and internalization by J774.2 cells. Cells were pretreated with inhibitors for 30 min at 37°C, and PGA (100 μg/well) or Alexa Fluor 555-labeled transferrin (5 μg/ml) was added and incubated for 1 h at 37°C. PGA bound to the cell surface was stained with Alexa Fluor 555-labeled MAb F24F2 (red), and interior PGA was stained with Alexa Fluor 488-labeled F26G3 MAb (green). The top panels show the effects of inhibitors on binding and uptake of PGA; the lower panels show the effects of inhibitors on the binding and uptake of transferrin. The results are representative of three experiments. PAO, phenylarsine oxide.
FIG. 5.
Effects of cytoskeletal inhibitors on PGA binding and uptake. J774.2 cells were preincubated for 30 min with either cytochalasin D (Cyto D), an actin inhibitor, or vinblastine (Vin), a microtubule inhibitor. In the top panels, PGA was added and incubated for 1 h. Exterior PGA was stained with Alexa Fluor 555-labeled MAb F24F2 (red), and interior PGA was stained with Alexa Fluor 488-labeled MAb F26G3 (green). In the bottom panels, GXM was added to the cells and incubated for 2 h. Exterior GXM was stained with Alexa Fluor 555-labeled MAb 3C2 (red), and interior GXM was stained with Alexa Fluor 488-labeled MAb 3C2. The results are representative of three independent experiments.
Degradation of PGA in macrophages.
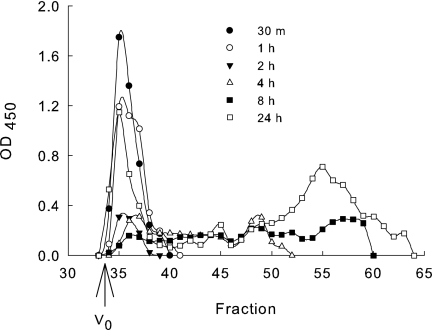
In previous studies, PGA was present in mouse urine as a heterogeneous mixture of low-molecular-weight fragments in comparison to native PGA (28). As a consequence, we wanted to examine macrophages as a possible source of PGA degradation. J774.2 cells were incubated with PGA for 0.5, 1, 2, 4, 8, and 24 h and lysed, and the relative molecular size of the PGA was evaluated by molecular sieve chromatography. Little or no PGA degradation was noted after 30 min, 1 h, and 2 h of incubation in comparison to a native PGA standard (Fig. 6). Degradation was observed after 4 h of incubation, and further degradation was noted after 8 and 24 h of incubation (Fig. 6). No degradation was noted if PGA was incubated for 30 min to 24 h with 2% paraformaldehyde-fixed cells (data not shown).
FIG. 6.
Degradation of PGA by J774.2 cells. J774.2 cells were incubated with PGA for the indicated time points. The cells were then lysed with 1% Tween 20 in water and centrifuged at 5,000 rpm for 10 min, and the PGA concentration in the supernatant fluids from the lysates was determined via antigen capture ELISA. PGA (50 ng) was run over a Superdex 200 molecular sieve column and the relative amount of PGA in each fraction is reported as the OD450 from an ELISA done with each fraction. V0 is the fraction at which the void volume elutes from the column. The OD450 on the y axis gives the relative concentration of detectable PGA and the fraction shows the relative molecular size. The data shown are from one of three separate experiments with similar results.
DISCUSSION
Bacillus anthracis is surrounded by an antiphagocytic capsule which is unique from other bacterial capsules in both composition and pharmacokinetics for in vivo clearance. While previous studies showed that the capsular material from B. anthracis can accumulate in resident macrophages in the liver and spleen (28), these studies did not address the details of PGA-macrophage interactions. To address this issue, we used a macrophage-like cell line, J774.2, to identify the course of uptake, the mechanisms utilized for uptake, and the intracellular fate of the capsular antigen. We found that PGA is taken up by J774.2 cells, localizes to CD71-positive endosomes throughout the time course tested, and shows signs of degradation after 4 h of incubation. These findings are somewhat unexpected because the majority of the PGA did not go to the lysosome for degradation. Following endocytosis, molecules are typically found in the early endosomes and then travel from the late endosomes to lysosomes (22). In addition to the traditional degradation pathway to the lysosome, several molecules and receptors such as the transferrin receptor and the low-density lipoprotein (LDL) receptors are trafficked back to the plasma membrane via the recycling early endosomes (6, 19). Although transferrin remains attached to its receptor while being trafficked through the recycling endosomes, LDL dissociates from its receptor in response to the acidification of the endosomes following endocytosis (6, 22).
Early endosomes, late endosomes, and lysosomes each have a distinct pH which allows for the dissociation of ligands from receptors, as well as the degradation of endocytosed molecules. Early endosomes have a pH of 6; late endosomes have a pH of 5 to 6; recycling endosomes have a pH of 6.4; and lysosomes have a more acidic pH of 5 (22, 29, 33). Lysosomes also have a high concentration of degradative enzymes; this is in contrast with early endosomes, which do not contain degradative enzymes (17). The degradation curves in Fig. 6 show that at the time points after 4 h, there are two distinct groups of PGA, one corresponding to PGA that elutes from the column at the void volume and one group that corresponds with PGA showing considerable degradation by elution from the column at later fractions. PGA present at the void volume fraction may be PGA that traffics through the recycling early endosomes where there are fewer degradative enzymes, whereas the low-molecular-weight PGA may be PGA that trafficked through the lysosomal degradation pathway.
We found that the degradation of PGA by J774.2 cells was apparent after 4 h of incubation and continued through 24 h of incubation. A caveat is that one of the obstacles to measuring smaller fragments of PGA by ELISA lies in constraints of the antigen capture ELISA. When a 25-mer synthetic polypeptide was used, the sensitivity of the assay was greatly reduced in comparison to that of the native PGA assay (28). Therefore, the sensitivity of the ELISA decreases with smaller molecular size. As a consequence, our results give us insight as to when PGA degradation occurs, but it is difficult to assess the true amount of PGA recovered from the cell lysates.
There has been little work done examining the intracellular fate of T-cell-independent capsular antigen from encapsulated pathogens. Studies done by Kaplan et al. (14), Goldman et al. (10), and Grinsell et al. (11) described the trafficking of capsular polysaccharides to tissues, urine, and serum in vivo but did not examine the intracellular fate. Chang et al. described the binding and kinetics of the uptake of GXM, the capsular material from C. neoformans, by peritoneal macrophages from BALB/c mice (2). They found that GXM begins to be internalized after about 15 min, which is slightly slower than the internalization of PGA in J774.2 cells (2). Although the initial uptake was slower, both studies noted that the interior and exterior antigen distributions were similar after 1 h of incubation (2). Although Chang et al. did not evaluate time points beyond 4 h, the general trends of the capsular uptake appeared to be similar for GXM and PGA, with PGA uptake occurring more rapidly (2).
Cobb et al. studied the capsular polysaccharides from Bacteroides fragilis (4). These studies found that zwitterionic polysaccharides, while contrasting with PGA in being T-cell-dependent antigens, are internalized in antigen-presenting cells in as little as 30 min. Internalization continued to 6 h, which coincides with the results we obtained for PGA uptake (4). Further study found that zwitterionic polysaccharide from B. fragilis localized to LAMP-1-positive lysosomes, and treatment with cytochalasin D reduced the uptake of capsular antigen (4).
Inhibitors are useful tools for examining the mechanisms of antigen uptake by macrophages. One of the major pathways that macrophages use to take up foreign particles and nutrients involves receptor-mediated endocytosis. Amantadine and phenylarsine oxide effectively block the receptor-mediated uptake of antigen by a variety of cell lines (9, 23, 27). Both amantadine and phenylarsine oxide blocked the binding and uptake of PGA in J774.2 cells. These findings highlight receptor-mediated pathways as one of the main mechanisms for the uptake of B. anthracis capsular antigen.
Cytochalasin D and vinblastine are classic cytoskeletal inhibitors used to study uptake pathways. Actin can play a critical role in receptor-mediated endocytosis (8, 16). As a consequence, we treated J774.2 cells with cytochalasin D, an inhibitor which caps actin filaments, thereby preventing assembly (24). The binding and uptake of PGA were partially inhibited at higher concentrations of cytochalasin D (2). This result is similar to that of previous studies with cryptococcal GXM; however, there was a complete blockade in the case of GXM (2). Microtubules also contribute to phagocytosis (20); therefore, we treated cells with vinblastine, a microtubule inhibitor. PGA was not inhibited by vinblastine at any of the concentrations examined. This result contrasts with the complete blockade of the binding and uptake of GXM by macrophages (Fig. 5) (2).
Our previous study of the in vivo pharmacokinetics for the clearance of PGA found that PGA accumulates in sinusoidal endothelial cells of the liver and macrophages in the liver and spleen (28). Our studies were limited to the uptake and processing of PGA by the macrophage cell line J774.2. The J774.2 cell line was used as a representative macrophage for the study of PGA uptake and processing. We cannot exclude the possibility that processing by sinusoidal endothelial cells may take a different course. Our studies found that PGA is both taken up and degraded by macrophages. Notably, the degradation of PGA by J774.2 macrophages is consistent with the degradation of PGA found in vivo (28). As a consequence, our results are relevant to the in vivo processing of PGA. However, further work is needed to compare the macrophage accumulation and clearance of PGA to the accumulation and processing of PGA by sinusoidal endothelial cells. Although PGA is a T-independent type 2 antigen (31), rapid degradation is not a feature that is commonly associated with this type of antigen (21). Finally, the degradation of PGA by macrophages in vivo may account for our previous finding that PGA is shed into urine (28). This latter finding will likely prove important in the event that an immunoassay for PGA proves useful in the diagnosis of anthrax (15).
Acknowledgments
This study was supported by public health service grants R01-AI059348 and U01-AI061200 from the National Institute of Allergy and Infectious Diseases. This study was also supported by the Graduate Student Association graduate research grant from the University of Nevada, Reno, NV.
Editor: A. Casadevall
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.Baravalle, G., D. Schober, M. Huber, N. Bayer, R. F. Murphy, and R. Fuchs. 2005. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res. 32099-113. [DOI] [PubMed] [Google Scholar]
- 2.Chang, Z. L., D. Netski, P. Thorkildson, and T. R. Kozel. 2006. Binding and internalization of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, by murine peritoneal macrophages. Infect. Immun. 74144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 5959-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobb, B. A., Q. Wang, A. O. Tzianabos, and D. L. Kasper. 2004. Polysaccharide processing and presentation by the MHCII pathway. Cell 117677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 42237-44. [DOI] [PubMed] [Google Scholar]
- 6.Davis, C. G., J. L. Goldstein, T. C. Sudhof, R. G. Anderson, D. W. Russell, and M. S. Brown. 1987. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature 326760-765. [DOI] [PubMed] [Google Scholar]
- 7.Drysdale, M., S. Heninger, J. Hutt, Y. Chen, C. R. Lyons, and T. M. Koehler. 2005. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 24221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto, L. M., R. Roth, J. E. Heuser, and S. L. Schmid. 2000. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1161-171. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, A. E., R. J. Noel, J. T. Herlihy, and W. F. Ward. 1989. Phenylarsine oxide inhibition of endocytosis: effects on asialofetuin internalization. Am. J. Physiol. 257C182-C184. [DOI] [PubMed] [Google Scholar]
- 10.Goldman, D. L., S. C. Lee, and A. Casadevall. 1995. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 633448-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinsell, M., L. C. Weinhold, J. E. Cutler, Y. Han, and T. R. Kozel. 2001. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J. Infect. Dis. 184479-487. [DOI] [PubMed] [Google Scholar]
- 12.Hanby, W. E., and H. N. Rydon. 1946. The capsular substance of Bacillus anthracis. Biochem. J. 40297-309. [PubMed] [Google Scholar]
- 13.Ivins, B. E., J. W. Ezzell, Jr., J. Jemski, K. W. Hedlund, J. D. Ristroph, and S. H. Leppla. 1986. Immunization studies with attenuated strains of Bacillus anthracis. Infect. Immun. 52454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan, M. E., A. H. Coons, and H. W. Deane. 1950. Localization of antigen in tissue cells; cellular distribution of pneumococcal polysaccharides types II and III in the mouse. J. Exp. Med. 9115-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozel, T. R., W. J. Murphy, S. Brandt, B. R. Blazar, J. A. Lovchik, P. Thorkildson, A. Percival, and C. R. Lyons. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA 1015042-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamaze, C., L. M. Fujimoto, H. L. Yin, and S. L. Schmid. 1997. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem. 27220332-20335. [DOI] [PubMed] [Google Scholar]
- 17.Lewin, B., L. Cassimeris, V. R. Lingappa, and G. Plopper. 2007. Cells. Jones and Bartlett Publishers, Sudbury, MA.
- 18.Lowy, R. J., and D. S. Dimitrov. 1997. Characterization of influenza virus-induced death of J774.1 macrophages. Exp. Cell Res. 234249-258. [DOI] [PubMed] [Google Scholar]
- 19.Marsh, E. W., P. L. Leopold, N. L. Jones, and F. R. Maxfield. 1995. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J. Cell Biol. 1291509-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGraw, T. E., K. W. Dunn, and F. R. Maxfield. 1993. Isolation of a temperature-sensitive variant Chinese hamster ovary cell line with a morphologically altered endocytic recycling compartment. J. Cell. Physiol. 155579-594. [DOI] [PubMed] [Google Scholar]
- 21.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13655-692. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77759-803. [DOI] [PubMed] [Google Scholar]
- 23.Ruckert, P., S. R. Bates, and A. B. Fisher. 2003. Role of clathrin- and actin-dependent endocytotic pathways in lung phospholipid uptake. Am. J. Physiol. Lung Cell. Mol. Physiol. 284L981-L989. [DOI] [PubMed] [Google Scholar]
- 24.Sampath, P., and T. D. Pollard. 1991. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry 301973-1980. [DOI] [PubMed] [Google Scholar]
- 25.Sandvig, K., M. Ryd, O. Garred, E. Schweda, P. K. Holm, and B. van Deurs. 1994. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol. 12653-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandvig, K., and B. van Deurs. 2002. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 52949-53. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel, R., R. B. Dickson, M. C. Willingham, and I. H. Pastan. 1982. Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin. Proc. Natl. Acad. Sci. USA 792291-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland, M. D., P. Thorkildson, S. D. Parks, and T. R. Kozel. 2008. In vivo fate and distribution of poly-γ-d-glutamic acid, the capsular antigen from Bacillus anthracis. Infect. Immun. 76899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tycko, B., and F. R. Maxfield. 1982. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell 28643-651. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Wang, T. T., and A. H. Lucas. 2004. The capsule of Bacillus anthracis behaves as a thymus-independent type 2 antigen. Infect. Immun. 725460-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welkos, S. L. 1991. Plasmid-associated virulence factors of non-toxigenic (pX01-) Bacillus anthracis. Microb. Pathog. 10183-198. [DOI] [PubMed] [Google Scholar]
- 33.Yamashiro, D. J., and F. R. Maxfield. 1987. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J. Cell Biol. 1052723-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]